Consider a fluorescent detector designed to report the cellular location of active protein tyrosine kinases. A blue
Question:
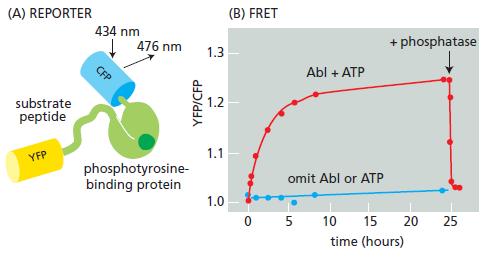
Consider a fluorescent detector designed to report the cellular location of active protein tyrosine kinases. A blue (cyan) fluorescent protein (CFP) and a yellow fluorescent protein (YFP) were fused to either end of a hybrid protein domain. The hybrid protein segment consisted of a substrate peptide recognized by the Abl protein tyrosine kinase and a phosphotyrosine-binding domain (Figure Q9–4A). Stimulation of the CFP domain does not cause emission by the YFP domain when the domains are separated. When the CFP and YFP domains are brought close together, however, fluorescence resonance energy transfer (FRET) allows excitation of CFP to stimulate emission by YFP. FRET shows up experimentally as an increase in the ratio of emission at 526 nm versus 476 nm (YFP/ CFP) when CFP is excited by 434 nm light. Incubation of the reporter protein with Abl protein tyrosine kinase in the presence of ATP gave an increase in YFP/CFP emission (Figure Q9–4B). In the absence of ATP or the Abl protein, no FRET occurred. FRET was also eliminated by addition of a tyrosine phosphatase (Figure Q9–4B). Describe as best you can how the reporter protein detects active Abl protein tyrosine kinase.
Figure Q9-4
Step by Step Answer:

Molecular Biology Of The Cell
ISBN: 9780815344322
6th Edition
Authors: Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter




