In a subset of voltage-gated K+ channels, the N-terminus of each subunit acts like a tethered ball
Question:
In a subset of voltage-gated K+ channels, the N-terminus of each subunit acts like a tethered ball that occludes the cytoplasmic end of the pore soon after it opens, thereby inactivating the channel. This “ball-andchain” model for the rapid inactivation of voltage-gated
K+ channels has been elegantly supported for the shaker
K+ channel from Drosophila melanogaster. (The shaker
K+ channel in Drosophila is named after a mutant form that causes excitable behavior—even anesthetized flies keep twitching.) Deletion of the N-terminal amino acids from the normal shaker channel gives rise to a channel that opens in response to membrane depolarization, but stays open instead of rapidly closing as the normal channel does. A peptide (MAAVAGLYGLGEDRQHRKKQ) that corresponds to the deleted N-terminus can inactivate the open channel at 100 μM.
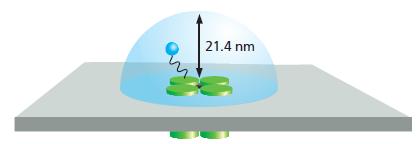
Is the concentration of free peptide (100 μM) that is required to inactivate the defective K+ channel anywhere near the local concentration of the tethered ball on a normal channel? Assume that the tethered ball can explore a hemisphere [volume = (2/3)πr3] with a radius of 21.4 nm, which is the length of the polypeptide “chain” (Figure Q11–2). Calculate the concentration for one ball in this hemisphere. How does that value compare with the concentration of free peptide needed to inactivate the channel?
Figure Q11-2
Step by Step Answer:

Molecular Biology Of The Cell
ISBN: 9780815344322
6th Edition
Authors: Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter





