Question: Using the results of the previous problem, show that for a harmonic wave with a phase Ï(x, t) = k(x - vt) we can determine
Using the results of the previous problem, show that for a harmonic wave with a phase φ(x, t) = k(x - vt) we can determine the speed by setting dφ/dt = 0. Apply the technique to Problem 2.32 to find the speed of that wave.
Data from Prob. 2.32
Use Eq. (2.33) to calculate the speed of the wave whose representation in SI units is
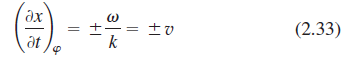
ψ(y, t) = A cos π(3 × 106y + 9 × 1014t)
(2.33) tv ot
Step by Step Solution
3.53 Rating (174 Votes )
There are 3 Steps involved in it
and this is zero provided dx... View full answer

Get step-by-step solutions from verified subject matter experts


