(A true story) The manager of an organic chemistry stockroom prepared unknowns for a Ketones and Aldehydes...
Question:
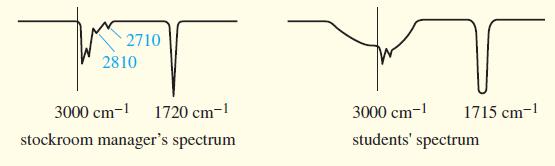
(A true story) The manager of an organic chemistry stockroom prepared unknowns for a “Ketones and Aldehydes” experiment by placing two drops of the liquid unknowns in test tubes and storing the test tubes for several days until they were needed. One of the unknowns was misidentified by every student. This unknown was taken from a bottle marked “Heptaldehyde.” The stockroom manager took an IR spectrum of the liquid in the bottle and found a sharp carbonyl stretch around 1720 cm-1 and small, sharp peaks around 2710 and 2810 cm-1.
The students complained that their spectra showed no peaks at 2710 or 2810 cm-1, but a broad absorption centered over the 3000 cm-1 region and a carbonyl peak around 1715 cm-1. They also maintained that their samples are soluble in dilute aqueous sodium hydroxide.
Step by Step Answer:






