(A true story.) While organizing the undergraduate stockroom, a new chemistry professor found a half-gallon jug containing...
Question:
(A true story.) While organizing the undergraduate stockroom, a new chemistry professor found a half-gallon jug containing a cloudy liquid (bp 100–105 °C), marked only “STUDENT PREP.” She ran a quick mass spectrum, which is printed below. As soon as she saw the spectrum (without even checking the actual mass numbers), she said, “I know what it is.”
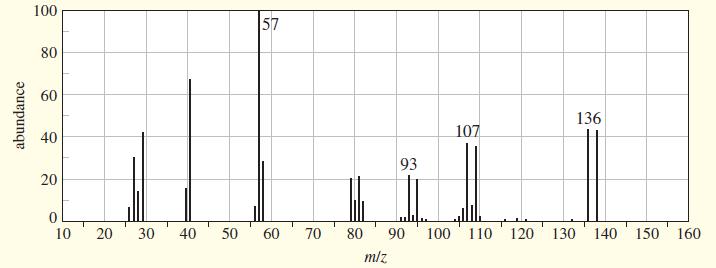
(a) What compound is the “student prep”? Any uncertainty in the structure?
(b) Suggest structures for the fragments at 136, 107, and 93. Why is the base peak (at m/z57) so strong?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





