Question: An important protecting group developed specifically for polyhydroxy compounds like nucleosides is the tetraisopropyldisiloxanyl group, abbreviated TIPDS, that can protect two alcohol groups in a
An important protecting group developed specifically for polyhydroxy compounds like nucleosides is the tetraisopropyldisiloxanyl group, abbreviated TIPDS, that can protect two alcohol groups in a molecule.
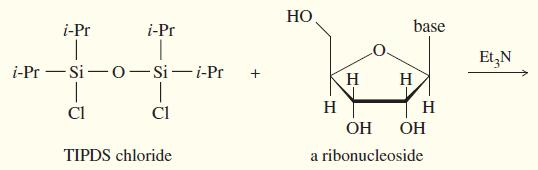
(a) The TIPDS group is somewhat hindered around the Si atoms by the isopropyl groups. Which OH is more likely to react first with TIPDS chloride? Show the product with the TIPDS group on one oxygen.
(b) Once the TIPDS group is attached at the first oxygen, it reaches around to the next closest oxygen. Show the final product with two oxygens protected.
(c) The unprotected hydroxyl group can now undergo reactions without affecting the protected oxygens. Show the product after the protected nucleoside from (b) is treated with tosyl chloride and pyridine, followed by NaBr, ending with deprotection with Bu4NF.
HO i-Pr i-Pr base Et,N i-Pr - Si -o-Si-i-Pr H H Cl Cl H H OH OH TIPDS chloride a ribonucleoside
Step by Step Solution
3.33 Rating (162 Votes )
There are 3 Steps involved in it
Since TIPDS chloride is a hindered protecting group it will protect first the hydroxyl ... View full answer

Get step-by-step solutions from verified subject matter experts


