Give IUPAC names for the following structures: (a) (d) O CH3 CH3CHCCH2CH3 T CH3 (b) CHO H-C-OH
Question:
Give IUPAC names for the following structures: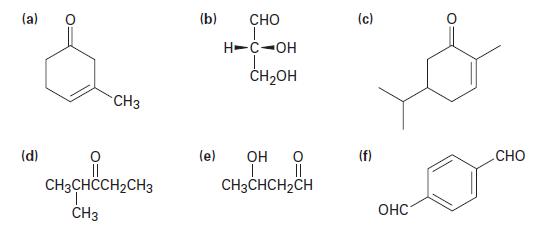
Transcribed Image Text:
(a) (d) O CH3 CH3CHCCH2CH3 T CH3 (b) CHO H-C-OH CH₂OH (e) OH O |_____ || CH3CHCH₂CH (c) (f) OHC O CHO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
a O This is a simple oxygen atom In IUPAC nomenclature it is not typically given a sp...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give IUPAC names for the following alkenes: (a) (b) (c) (d) (e) (f) OH CI
-
Give IUPAC names for the following compounds. (a) (b) (c) (d) (e) (f) Ph CH3C C CH CH H3C CH3 - (CH3)3C C-CH (CH3)CH2CH3 CH CHC CC-OH CH,CH CH C-C CH
-
Give IUPAC names for the following compounds. (a) (b) (c) CH3 CH CH CH CH, CH, CH CH
-
Each table of values gives several points that lie on a line.(a) What is the x-intercept of the line? The y-intercept?(b) Which equation in choices AD corresponds to the given table of values?(c)...
-
White, Inc., had depreciation expenses on its plant assets as follows for 2009, 2010, and 2011, respectively: $267,000, $289,000, and $357,000. Compute the trend percentages for these years, assuming...
-
During a certain part of the day, an average of five automobiles arrives every minute at the tollgate on a turnpike. Let X be the number of automobiles that arrive in any 1-minute interval selected...
-
Why are each of the BRIC countries viewed as potential candidates for global expansion?
-
When a flammable liquid (such as gasoline or charcoal lighter fluid) ignites, it is not the liquid itself that burns: what actually happens is that the liquid vaporizes and the resulting airvapor...
-
Required: Using the data provided, compute the margin of safety and margin of safety ratio
-
In light of your answer to Problem 9.35, what stereochemistry would you expect the product from the reaction of phenylmagnesium bromide with butan-2-one to have? Problem 9.35 Show the structures of...
-
What product would you expect from nucleophilic addition of methanol, CH 3 OH, to benzaldehyde under acidic conditions?
-
You are an executive at Procter and Gamble and are about to introduce a new product. Your boss has asked you to predict the market share (Q, a proportion between 0 and 1) that the new product will...
-
The composition of moist air is given on a molar basis to be 78 percent N2, 20 percent O2, and 2 percent water vapor. Determine the mass fractions of the constituents of air. Use the table containing...
-
1. Consider the LFSR with so = 1, 8 = 1, S2 = 1, 83 = 1, 84 = 0, and Sn Sn-2 Sn-3+ Sn-5. Find the next 15 terms in this LFSR. What is the period of this LFSR? 2. Suppose you learn that a Hill cipher...
-
Assume that you are thinking of a new acquisition campaign for SEDO, assuming that you want to convert people who are already engaged. Develop a big idea (in the communication) that you can use in...
-
You have a backend Amazon EC2 instance providing a web service to your web server instances. Your web servers are in a public subnet. You would like to block inbound requests from the internet to...
-
Consider the following task set. Task C T|D T1 20 50 40 T2 10 40 30 T3 5 20 15 a) Verify whether the task set is schedulable under DM using the processor utilization-based ap- proach. b) Verify...
-
David's utility function is U = q1 + 2q2. Describe his optimal bundle in terms of the prices of q1 and q2?
-
Phosgene, COCl2, is a toxic gas used in the manufacture of urethane plastics. The gas dissociates at high temperature. At 400oC, the equilibrium constant Kc is 8.05 104. Find the percentage of...
-
Propose the structure for a compound that lacks a methyl group but nevertheless exhibits a quartet in its 1 H NMR spectrum.
-
Each of the three vinylic protons of styrene is split by the other two, and the J values are found to be J ab = 11 Hz, Jac = 17 Hz, and J bc = 1 Hz. Using this information, draw the expected...
-
Draw the expected 1 H NMR spectrum for each of the following compounds: (a) (b) (c) H.
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


