How many signals would you expect to see in the 1 H NMR spectrum of each of
Question:
How many signals would you expect to see in the 1H NMR spectrum of each of the following compounds?
a. CH3CH2CH2CH3
b. BrCH2CH2Br
c. CH2=CHCl
d.
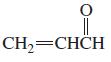
e.
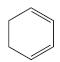
f.
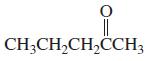
g.
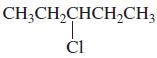
h.
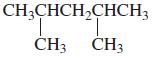
i.
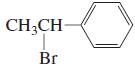
j.
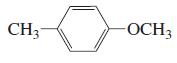
k.
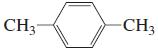
l.
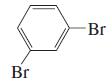
m.
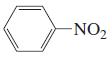
n.
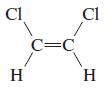
o.
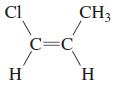
Transcribed Image Text:
CH2=CHCH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
a 2 signals 6 hydrogen on terminal CH3 group forms a signal and the hydrogen of CH2 grou...View the full answer

Answered By

DIPIN DAS AK
I completed my masters in chemistry from CUSAT, kerala, India. I studied bachelor of science from Calicut University and i had a project in nanotechnology from Hyderabad central university. I have two year teaching experience in higher secondary level in India as a private tutor. Now i work as a subject matter expert in conects app for part time. I love teaching and sharing knowledge.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How many signals would you expect in the 1 H NMR spectrum of each of the following compounds: (a) (b) (c) (d) (e) (f) CI Br -
-
How many signals would you expect to find in the 1H NMR spectrum of each of the following compounds? (a) 1-Butanol (b) Butane (c) 1,4-Dibromobutane
-
How many 1H NMR signals would you expect from each of the following compounds? (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (l) (m) (n) (o) (p) OH CI
-
What other cost factors might you include in such an economic analysis? The proposed small office building in Example 3-2 has 24,000 net square feet of area heated by a natural gas furnace. The owner...
-
Light passes from benzene (medium 1) to water (medium 2) in Figure 19-19 at (a) 1 = 30 or (b) 1= 0. Find the angle 2 in each case.
-
. Would you describe the Container Store as a highperformance organization? Why or why not?
-
Pain tolerance study. A study published in Psychosomatic Medicine (Mar./Apr. 2001) explored the relationship between reported severity of pain and actual pain tolerance in 337 patients who suffer...
-
J. Ackbar, a former professional tennis star, operates Ackbars Tennis Shop at the Miller Lake Resort. At the beginning of the current season, the ledger of Ackbars Tennis Shop showed Cash $2,500;...
-
You have entered into an agreement to purchase a local accounting firm. The agreement specifies you will pay the seller $192,000 each year for six years. What is the cost today of the purchase,...
-
Tony and Suzie see the need for a rugged all-terrain vehicle to transport participants and supplies. They decide to purchase a used Suburban on July 1, 2025, for $12,000. They expect to use the...
-
Which of the following compounds has a vibration that is infrared inactive: acetone, 1-butyne, 2-butyne, H 2 , H 2 O, Cl 2 , ethene?
-
How could you distinguish the 1 H NMR spectra of the following compounds? a. CH 3 OCH 2 OCH 3 b. CH 3 OCH 3 c. CH3 CH3OCH,CCH2OCH3 H3
-
What specific information or evidence can an IT auditor gather for a client that uses its IT environment to store and process financially significant data?
-
5.Descibe the HSI color image model 6. Describe the basic relationship between the pixels
-
1. What is the need for transform? 2. What is Image Transform? 3. What are the applications of transform? 4. Give the Conditions for perfect transform . 5. What are the properties of unitary...
-
6. Define Fourier transform pair 7. Define Fourier spectrum and spectral density 8. Give the relation for 1-D discrete Fourier transform pair 9. Specify the properties of 2D Fourier transform. 10....
-
16. What is wrap around error? 17. Give the formula for correlation of 1D continuous function. 18. What are the properties of Haar transform. 19. What are the Properties of Slant transform 20....
-
21. Define fast Walsh transform. 22. Give the relation for 1-D DCT. 23. Write slant transform matrix SN. 24. Define Haar transform. 25. Define K-L transform. 26. Give the equation for singular value...
-
Change from rectangular to spherical coordinates. (a) (0, 4, 4) (b) (2, 2, 26)
-
Draw and label the E and Z isomers for each of the following compounds: 1. CH3CH2CH==CHCH3 2. 3. 4. CH,CH2C CHCH2CH Cl CH3CH2CH2CH2 CH CH2CCCH2CI CHCH3 CH3 HOCH CH CCC CH O-CH C(CH
-
(a) What type of pericyclic reaction is required to form benzene from Dewar benzene? (b) Explain why Dewar benzene, although a very un-stable molecule, is not readily transformed to benzene....
-
Show both conrotatory processes for the thermal electtrocyclec conversion of (2E, 4E) - 2,4 -hexadiene into 3,4- dimethylcyclobutene (Eq. 27.8 ). Explain why the two processes are equally likely.
-
Show both conrotatory processes for the thermal electtrocyclec conversion of (2E, 4E) - 2,4 -hexadiene into 3,4- dimethylcyclobutene (Eq. 27.8 ). Explain why the two processes are equally likely.
-
Javier is currently paying $1,200 in interest on his credit cards annually. If, instead of paying interest, he saved this amount every year, how much would he accumulate in a tax-deferred account...
-
Your company is considering the purchase of a fleet of cars for $195,000. It can borrow at 6%. The cars will be used for four years. At the end of four years they will be worthless. You call a...
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...

Study smarter with the SolutionInn App


