How would you prepare the following ketones by reaction of a Grignard reagent and a nitrile? (a)
Question:
How would you prepare the following ketones by reaction of a Grignard reagent and a nitrile?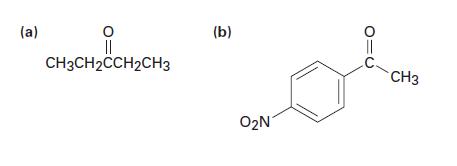
Transcribed Image Text:
(a) || CH3CH2CCH2CH3 (b) O₂N CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
To prepare ketones using a Grignard reagent and a nitrile you would follow these steps a CH3CH2CCH2C...View the full answer

Answered By

User l_917591
As a Business Management graduate from Moi University, I had the opportunity to work as a tutor for undergraduate students in the same field. This experience allowed me to apply the theoretical knowledge I had gained in a practical setting, while also honing my teaching and communication skills.
As a tutor, I was responsible for conducting tutorial sessions, grading assignments and exams, and providing feedback and support to my students. I also assisted with the preparation of course materials and collaborated with other tutors and professors to ensure consistency in teaching and assessment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
2. What is the most important difference between generic software product development and custom software development? What might this mean in practice for users of generic software products? (2...
-
On 1 April 2019, Lisa borrowed $47204 at an interest rate of 2.37% from her employer, Solarcity Pty Ltd. This was lower than that offered to the public. Lisa uses the entire amount to replace the...
-
The Reformatsky reaction is an addition reaction in which an organozinc reagent is used instead of a Grignard reagent to attack the carbonyl group of an aldehyde or a ketone. Because the organozinc...
-
In Problems 1118, mentally solve each equation. 6x = -24
-
The contribution margin ratio of the Furniture Department at Glad's Mercantile is 0.75. The trace able fixed costs for the Furniture Department are estimated at $188,000 per year. Sales in the...
-
Find the arclength of the graph given below. (Round your answer to four decimal places.) y = In(sin(x)); x 4
-
Compare the distribution of responses by females with the national distribution. What can you conclude?
-
Effect of order quantity on special order decision Lang Company made 100,000 electric drills in batches of 1,000 units each during the prior accounting period. Normally, Lang markets its products...
-
Identify characteristics of specific accounts. Asset Company Owns? Y or N Liabilities Company Pays to Someone Else? Y or N Effect on Owner's Equity: Increase, Decrease, or NA Equation Element: Asset,...
-
Show the enolate ions you would obtain by deprotonation of the following carbonyl compounds: (a) 0 CH3CHCHCH (b) 0 CH3CCHCH3 (c) 0 CH3
-
Show the products of hydrolysis of the following esters: (a) 0 CH3 || | CH3COCHCH3 (b) COCH3
-
Bega Ltd was registered as a new company on 2 January 2019. On that day a prospectus was issued inviting applications for 300 000 ordinary shares at $10, payable $2.50 on application, $2.50 on...
-
Complete Exercises 2-B and 2-H in Writing and Analysis in the Law using what you learned in the reading and in the Seminar. Use paragraph form, use complete sentences, and make sure you use proper...
-
What is the value of a stock expected to be in 9 years if the annual dividend is expected to remain unchanged forever at $3.65, the expected rate of return is 6.9% per year, and the next dividend is...
-
Once invested IN a corporation, shareholders want their money out - they want a return on investment! John owns 2 5 % of REFUND CORP INC, which paid out a $ 5 0 , 0 0 0 distribution to him on 1 2 / 3...
-
Worksheet Financial Statement Ratios. Lowe's Companies, Inc Jan 28, 2022 and Jan. 29, 2021 Current Ratio Current Assets / Current Liabilities Acid Test Current Assets Current Liabilities (Cash + ST...
-
3. Peter Senen operates in a JIT manufacturing system. For August, Peter Senen purchased 10,000 units of raw materials at P1.00 per unit on account.What is the The journal entry to record the...
-
Are relatively more high-quality navel oranges sold in California or in New York? Why?
-
Element compound homogeneous mixture (heterogeneous mixture) 4) A piece of gold has a mass of 49.75 g. What should the volume be if it is pure gold? Gold has a density of 19.3 g/cm (3 points) D=m/v...
-
Consider the following two compounds: a) Identify which of these two compounds has greater resonance stabilization. b) Would you expect compound C (below) to have a resonance stabilization that is...
-
In each case below, identify the acid and the base. Then draw the curved arrows showing a proton transfer reaction. Draw the products of that proton transfer, and then predict the position of...
-
Identify the reagents you would use to accomplish each of the following transformations: Br . - Br Br En En
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


