Predict the splitting patterns for each proton in the following molecules: (a) CHBr2CH3 (d) || CH3CHCOCHCH3
Question:
Predict the splitting patterns for each proton in the following molecules: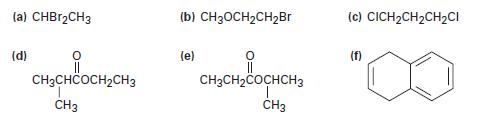
Transcribed Image Text:
(a) CHBr2CH3 (d) 유 || CH3CHCOCH₂CH3 CH3 (b) CH3OCH₂CH₂Br (e) CH3CH₂COCHCH3 CH3 (c) CICH₂CH₂CH₂CI (f)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
aWhen a hydrogen atom is attached to a terminal CH2 group the adjacent carbon carries one hydrogen T...View the full answer

Answered By

Fari Ahsan
As a tutor, I have extensive hands-on experience and a high level of proficiency in different areas of Mathematics. I have successfully guided students in various subjects like in chemical engineering , provided personalized instruction, and adapted teaching methods to suit individual learning styles. My expertise includes fostering critical thinking, problem-solving, and effective communication skills to promote academic growth. As a tutor, I'm passionate about helping my students achieve their academic goals and supporting them in their learning journey. I have tutored for 2 years in offline tutions and helped hundreds of students. Now looking forward to work in this platform.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the splitting patterns for the signals given by each of the compounds in Problem 3. a. CH3CH2CH2CH3 b. BrCH2CH2Br c. CH2==CHCl d. e. f. g. h. i. j. k. l. m. n. o. CH,-CHCH CH.CH.CH.CCH CH,CH...
-
Predict the splitting patterns you would expect for each proton in the followingmolecules: (b) CH2H2Br (a) CHBr2CH3 (c) CICH,CH2CH2CI (e) CHCH-CHCH (f) (d) CHCH2CH CH ondeo CH
-
Construct a simulated 1H NMR spectrum, including proton integrations, for CH3OC(CH2OCH3)3 ). Drag the appropriate splitting patterns to the approximate chemical shift positions; place the integration...
-
Investigate the recycling policy in the city where you live. How might you interpret the policy using the concepts or policy criteria presented in this chapter?
-
The catering manager of LaVista Hotel, Lisa Ferguson, is disturbed by the amount of silverware she is losing every week. Last Friday night, when her crew tried to set up for a banquet for 500 people,...
-
Which of the following controls would be least effective in ensuring that the correct product is shipped and billed at the approved price? a. Self-checking digits are used on all product numbers, and...
-
20-3. Cul es la principal diferencia entre un tomador de pedidos y un recolector de pedidos ?
-
Taft Corporation operates primarily in the United States. However, a few years ago it opened a plant in Spain to produce merchandise to sell there. This foreign operation has been so successful that...
-
Alyeski Tours operates day tours of coastal glaciers in Alaska on its tour boat the Blue Glacier. Management identified two cost drivers for budgeting purposes - the number of cruises and the number...
-
Show the products of each of the following reactions: (a) (b) (c) || CH3CHCHCHCHCSCOA Product of (a) + HO Product of (b) FAD FADH2 Acyl-CoA dehydrogenase Enoyl-CoA hydratase NAD+ NADH/H+*...
-
What enzyme cofactor is associated with transamination?
-
a. Draw the hierarchy chart and then plan the logic for a program that calculates a persons body mass index (BMI). BMI is a statistical measure that compares a persons weight and height. The program...
-
On Apple company with specific iPhone product Required to conduct a SWOT and PESTEL analysis, identifying the internal strengths and weaknesses and external opportunities and threats of the Apple...
-
In which social platforms are Walmart's brand/company active? In your opinion, are they doing a good job regarding customer engagement through social media channels? (Required: screenshots from the...
-
After you have watched both films, how would you describe each film? Also, consider what makes these early films different. List as many observations as you can that separate the Lumi re brothers...
-
How to develop the following points with the Poshmark application for second hand? 1. What are the main reasons for using this product? Or why not? 2. What are the hidden motivations? 3. Are there...
-
Suppose, in an experiment to determine the amount of sodium hypochlorite in bleach, you titrated a 22.84 mL sample of 0.0100 M K I O 3 with a solution of N a 2 S 2 O 3 of unknown concentration. The...
-
In messages to customers, what's wrong with words such as complaint, criticism, defective, failed, mistake, and neglected?
-
Consider the function f and its graph. a. Estimate the zeros of the area function b. Estimate the points (if any) at which A has a local maximum or minimum. c. Sketch a graph of A, for 0 x 10,...
-
What is the physical origin of the pressure difference across a curved liquidgas interface?
-
Draw the mechanism for each of the following transformations: a. b. c. Br HBr
-
Using the result of Equation (3.8), (P/T) V = / , express as a function of and V m for an ideal gas, and as a function of b, , and V m for a van der Waals gas.
-
If John invested $20,000 in a stock paying annual qualifying dividends equal to 4% of his investment, what would the value of his investment be 5 years from now? Assume Johns marginal ordinary tax...
-
help asap please!
-
Please, help asap! I have one day. Feedback will be given. & show some work. [in Excel] For the final project you will need you to create a spreadsheet /proforma of the cash flows from a property....

Study smarter with the SolutionInn App


