Show how you might synthesize the following tertiary amine three different ways, each using a different secondary
Question:
Show how you might synthesize the following tertiary amine three different ways, each using a different secondary amine and adding the final substituent by
(a) Reductive amination (3 ways).
(b) Acylation–reduction (3 ways).
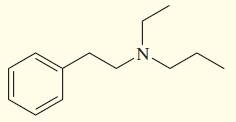
Transcribed Image Text:
N.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
aCondensation of the secondary amine with a carbonyl group ie an ald...View the full answer

Answered By

Thiruvengadam Vedamurthy
I have completed MPhil in Organic chemistry and MSc in chemistry. I am teaching for X, XI and XIIth CBSE as well as state board syllabus for more than two years. Currently , I am teaching chemistry for the NEET competitive examination.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Show how you might synthesize each of the following compounds using, as your starting materials, esters, ketones, acyl halides, and so on: (a) (b) (c) OEt OEt
-
Show how you might synthesize each of the following compounds from 1-butanol: (a) Butylamine (free of 28 and 38 amines) (b) Pentylamine (c) Propylamine (d) Butylmethylamine
-
Show how you might synthesize each of the following starting with a-tetralone (Section 15.9): (a) (b) (c) (d) HO So OH 02 H5
-
Bryce owns 200 shares of Basic Company stock that he purchased for $8,000 three years ago. On December 28, 2021, Bryce sold 100 shares of the stock for $2,500. On January 3, 2022, Bryce repurchased...
-
Explain why the total solubility of lead in Figure 6-3 first decreases and then increases as [ I-] increases. Give an example of the chemistry in each of the two domains.
-
Calculating Returns Suppose a stock had an initial price of $75 per share, paid a dividend of $1.20 per share during the year, and had an ending share price of $86. Compute the percentage total...
-
From calls made with randomly generated telephone numbers, 1012 respondents are asked if they rent or own their residences.
-
1. John Hood claims that he has no power or authority in his job. Is he correct? What sources of power work for and against him during this change process? 2. What influence tactics has Hood used...
-
The information that follows relates to equipment owned by Sweet Acacia Limited at December 3 1 , 2 0 2 3 :Crane Corp. acquired 6 , 4 0 0 shares of Mandrake Corp. on July 1 , 2 0 2 4 at $ 9 per...
-
Redstone Mill is a manufacturer that makes all sales on 30-day credit terms. Annual sales are approximately $40 million. At the end of 2014, accounts receivable were presented in the company's...
-
A compound of formula C 11 H 16 N 2 gives the IR 1 H NMR, and 13 C NMR spectra shown. The proton NMR peak at 2.0 disappears on shaking with D 2 O Propose a structure for this compound, and show how...
-
Propose mechanisms for the nucleophilic acyl substitutions to form ethyl benzoate and N-methylacetamide as shown on the previous page.
-
Refl ect on a recent meeting you have attended or a group conversation you have participated in. What role (or roles) did you take in the conversation (moving, opposing, following, or bystanding)...
-
Refer to Figure 11.2: Is it more costly to build in Los Angeles or in Washington DC? What is the cost difference? Figure 11.2 Location Factors Costs shown in RSMeans Square Foot Costs are based on...
-
Suppose the prism in Figure P33.27 is immersed in a liquid in which the speed of light is lower than the speed of light in glass. Describe what happens to the light shown entering at normal...
-
Each year, the AICPA issues a general audit risk alert document and a number of industry audit risk alerts. If you can obtain access to a current copy of either the general alert or one of the...
-
The multieffect distillation system shown in Figure 11-4 appears to be able to cut energy use in half; however, the reduction is not this large. Explain why. Figure 11-4 F PL D, D Reflux B PH
-
Schemes 11-6E and 11-6F accomplish the same task of removing and purifying an intermediate component. a. What factors enter into the decision to use scheme \(11-6 \mathrm{~F}\) instead of \(11-6...
-
At the instant under consideration, the hydraulic cylinder AB has a length L = 0.75 m, and this length is momentarily increasing at a constant rate of 0.2 m /s. If v A = 0.6 m /s and = 35, determine...
-
How does health insurance risk differ from other types of insurance risk (e.g., automobile or homeowners insurance)? What is the difference between cost sharing and cost shifting? Is retiree health...
-
Give the principal product(s) expected when 4-methyl-cyclohexene or other compound indicated reacts under the conditions in Problem 17.18. (a) Br2 in CH2Cl2, dark (b) A-bromosuccinimide in CCl4,...
-
Identify the benzylic carbons in the following structures. H,C-
-
A student Al Lillich has prepared a pure sample of 3-bromo-l-butene (A). Several weeks later he finds that the sample is contaminated with an isomer B formed by allylic rearrangement. (a) Give the...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


