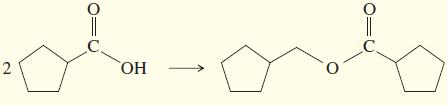
Show how you would accomplish the following multistep syntheses. You may use any additional reagents and solvents
Question:
Show how you would accomplish the following multistep syntheses. You may use any additional reagents and solvents you need.
a. PhCH2CH2OH → PhCH2CH2COOH
b.
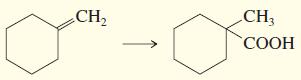
c.
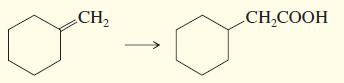
d.
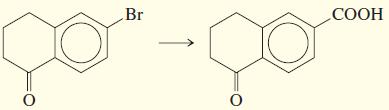
e.
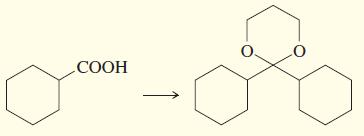
f.
Transcribed Image Text:
CH3 CH2 СООН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
a b c d e f Ph...View the full answer

Answered By

AKASH PANJA
I'm currently in 4th semester of Msc organic chemistry. I'm doing MSc from Central University Of Haryana, India. I've done two research Projects from Centre Of Biomedical Research (CBMR), Lucknow, India in organocatalysis and metal Catalyzed Asymmetric synthesis.
I'm a former subject expert in chegg. I can solve any questions of chemistry with a very detailed explanations in a cheap rate compare to others.
https://www.linkedin.com/in/akash-panja-516247196
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Show how you would accomplish the following synthetic conversions. You may use any additional reagents and solvents you need. (a) (b) (c) (d) Ph-CHO OH PhCHOPhCHC CCH2CH3
-
Show how you would accomplish the following multistep conversions. You may use any additional reagents you need. a. b. c. d. dimethyl adipate and allyl bromide
-
Show how you would accomplish the following syntheses. You may use whatever additional reagents you need. (a) (b) (c) (d) (e) (f) CHO CH,OH CHO CHO CH,Br Ph of CH, CH cth CH CH CHC-Ph 0 BrCH,CH,CCH...
-
Use any method to determine if the series converges or diverges. Give reasons for your answer. n10 10" n=1
-
Find [Cu2+] in a solution saturated with Cu4(OH)6(SO4) if [OH-1] is fixed at 1.0 10-6M. Note that Cu4(OH)6(SO4) gives 1 mo of SO42- for 4 mol of Cu2+. Ksp = 2.3 10-69
-
Nonconstant Growth and Quarterly Dividends Pasqually Mineral Water, Inc., will pay a quarterly dividend per share of $.80 at the end of each of the next 12 quarters. Thereafter, the dividend will...
-
The average age of the 115 residents of a retirement community
-
Justification/Recommendation Report: Improving Greenhouse Markets Service You are a recently hired manager for Greenhouse Market, a highend fast-food restaurant that has been in business for three...
-
Bill Blank signed an $8,900 note at Citizens Bank. Citizens charges a 3.4% discount rate. Assume the loan is for 260 days. A Find the proceeds. (Use 360 days a year. Round your intermediate...
-
Draw an AON network using the following data and find the probability of completing the critical path of the operatic project in 44 days, the official openingdate. b-420743342423 3 010531222312...
-
When pure (S)-lactic acid is esterified by racemic butan-2-ol, the product is 2-butyl lactate, with the following structure: (a) Draw three-dimensional structures of the two stereoisomers formed,...
-
The following NMR spectra correspond to compounds of formulas (A) C 9 H 10 O 2 , (B) C 4 H 6 O 2 , and (C) respectively. Propose structures, and show how they are consistent with the observed...
-
In Exercises, identify the open intervals on which the function is increasing or decreasing. h(x) = (x + 2)1/3 + 8
-
Safeway, Inc., operated 1,739 stores as of January 3, 2009. The following data were taken from the company's annual report. All dollar amounts are in thousands. Required a. Compute Safeway's...
-
Rich French, the owner of Rich's Fishing Supplies, is surprised at the amount of actual inventory at the end of the year. He thought there should be more inventory on hand based on the amount of...
-
Carol Lapaz owned a small company that sold boating equipment. The equipment was expensive, and a perpetual system was maintained for control purposes. Even so, lost, damaged, and stolen merchandise...
-
The following footnote related to accounting for inventory was taken from the 2008 annual report of Wal-Mart, Inc. Inventories The Company values inventories at the lower of cost or market as...
-
Plot the magnitude and phase of the frequency response of normalized n-th order lowpass Butterworth filters.
-
Refer to Exercise 5.45 and the Analytical Chemistry (Dec. 15, 2009) study in which scientists used high-performance liquid chromatography to determine the amount of drug in a tablet. Twenty-five...
-
Discuss the concept of the looking-glass self. how do you think others perceive you? do you think most people perceive you correctly?
-
Propose a curved-arrow mechanism for the following reaction. Explain why the equilibrium lies to the right. Ph Ph CH toluenesulfonic acidCH
-
Why is trityl chloride much more reactive than the other alkyl halides in Table 17.2? TABLE 17 2 comparison of S,1 Solvolysis Rates of Benzylic and Nonbenzylic Alkyl Halides 25C R-CIHo l + H2O -OH +...
-
What product(s) are expected when each of the following compounds reacts with one equivalent of NBS in CC14 in the presence of light and peroxides? Explain your answers. (a) cyclohexene (b)...
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


