Tamoxifen and clomiphene have similar structures but very different medical uses. Tell whether the alkene double bond
Question:
Tamoxifen and clomiphene have similar structures but very different medical uses. Tell whether the alkene double bond in is E or Z.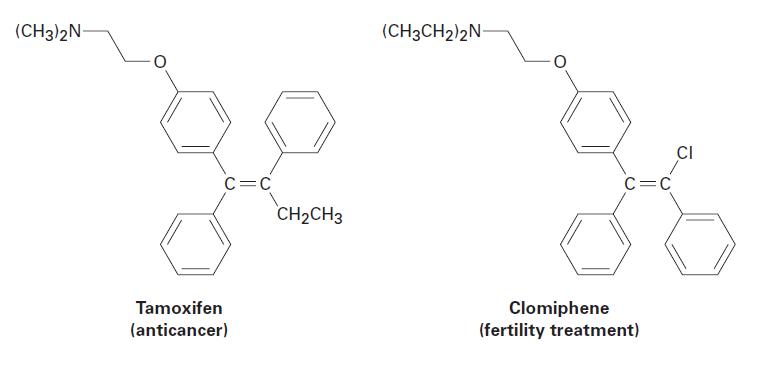
Transcribed Image Text:
(CH3)2N- C=C Tamoxifen (anticancer) CH₂CH3 (CH3CH2)2N- C=C Clomiphene (fertility treatment) CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
To determine whether the alkene double bond in the structure is E or Z we need to examine the substi...View the full answer

Answered By

User l_1006857
I am a computer science professional with expertise in databases, AI programming, data structures and algorithms, and mathematics. With a strong background in these areas, I possess the knowledge and skills necessary to design and optimize database systems, develop intelligent algorithms and models, and solve complex computational problems. My proficiency in SQL, NoSQL, machine learning techniques, and mathematical concepts equips me to contribute to innovative projects and drive technological advancements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
Ballard Ltd makes three products A, B and C. Each passes throughtwo departments: Machining and Assembly. Budgeted production ineach department by each productUnitsMachiningAssemblyPr 2 answers
-
Journalize the following transactions for The Hobby Hut, Inc., that occurred during the month of December. Dec 3 Purchased $6,500 of merchandise on account, terms 1/10, n/30, FOB shipping point. The...
-
A researcher conducts a 2 2 4 ANOVA. How many interaction effects are possible?
-
What is meant by the spread? What are the two measures of it? LO.1
-
Economist Lester Thurow once posed the following question: If you were the president of your own country and could choose one of two industries in which to specialize, computer chips or potato chips,...
-
maury bought one full bitcoin
-
Retin A, or retinoic acid, is a medication commonly used to reduce wrinkles and treat severe acne. How many different isomers arising from double bond isomerizations are possible? Retin A...
-
Change the following old names to new, post-1993 names, and draw the structure of each compound: (a) 2,5,5-Trimethyl-2-hexene (b) 2,2-Dimethyl-3-hexyne (c) 2-Methyl-2,5-heptadiene (d)...
-
Jackie Iverson was furious. She was about ready to fire Tom Rich, her purchasing agent. Just a month ago, she had given him a salary increase and a bonus for his performance. She had been especially...
-
The air in an automobile tire with a volume of \(0.015 \mathrm{~m}^{3}\) is at \(30^{\circ} \mathrm{C}\) and \(140 \mathrm{kPa}\) (gage). Determine the amount of air that must be added to raise the...
-
Convex Productions has just received a contract to film a commercial video that will air during a major sporting event in North America, and then be available on-demand through banner advertisements...
-
The following data (and annotations) for March 2016 are for the work in process account of the first of Olympus Companys four departments used in manufacturing its nly product. Assuming that Olympus...
-
If relative volatility can be assumed constant over the change in concentration for each fraction, Eq. \((9-13)\) can be adapted to the collection of fractions from a simple binary batch...
-
(a) Design a PI controller for Problem 8.6-4(b). (b) Design a PD controller for Problem 8.6-4(c). (c) Use the results of parts (a) and (b) to repeat Problem 8.6-4(d). Problem 8.6-4(b) (c) (d) (b)...
-
Cassidee owns a condominium near Newport Beach in California. This year, she incurs the following expenses in connection with her condo: Insurance......................................$1,500 Mortgage...
-
Fill in each blank so that the resulting statement is true. 83 + 103 = ______ .
-
(a) Compute the heats of reaction for abstraction of a primary hydrogen and a secondary hydrogen from propane by a fluorine radical. (b) How selective do you expect free-radical fluorination to be?...
-
2,3-Dimethylbutane reacts with bromine in the presence of light to give a monobrominated product. Further reaction gives a good yield of a dibrominated product. Predict the structures of these...
-
In the presence of a small amount of bromine, cyclohexene undergoes the following lightpromoted reaction: (a) Propose a mechanism for this reaction. (b) Draw the structure of the rate-limiting...
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


