Question: Tell whether the two structures in each pair represent enantiomers or two molecules of the same compound in different orientations. a. b. c. Br. CI
Tell whether the two structures in each pair represent enantiomers or two molecules of the same compound in different orientations.
a.
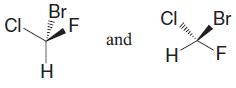
b.

c.
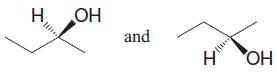
Br. CI Br Cl I F and H F H
Step by Step Solution
3.39 Rating (171 Votes )
There are 3 Steps involved in it
a Enantiomer... View full answer

Get step-by-step solutions from verified subject matter experts


