Tetracaine, a substance used as a spinal anesthetic, can be prepared from benzene by the following route.
Question:
Tetracaine, a substance used as a spinal anesthetic, can be prepared from benzene by the following route. Show how you could accomplish each of the transformations (a) through (d).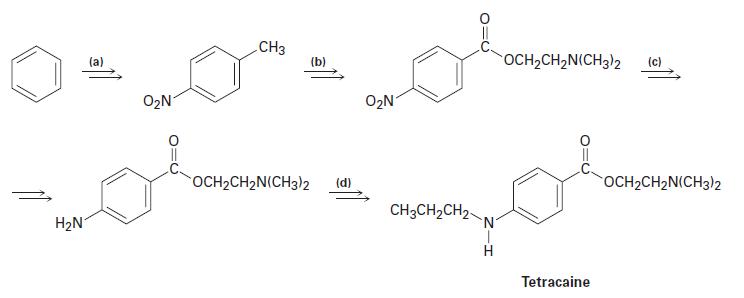
Transcribed Image Text:
H₂N (a) O₂N CH3 (b) OCH₂CH₂N(CH3)2 (d) O₂N CH3CH₂CH₂- N OCH₂CH₂N(CH3)2 Tetracaine OCH₂CH2N(CH3)2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
nhn nhiu nhiu nhiu c nhiu chu nhiu ...View the full answer

Answered By

Samee Ullah
Algebra, Linear algebra, calculus, accounting, marketing, statistics, programming, real estate, writing, human resource management, business communication, Engineering: civil, chemical, electrical, mechanical, aerospace, building
Linguistics: sociolinguistics, applied linguistics, music, social sciences, biology, chemistry: all types, Thermodynamics, mechanics, modern physics, quantum physics, metaphysics, biology.
Feel free to contact us for all these subjects,; for quality, and best responses. Thankyou
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Show how you could prepare each of the following amines from benzaldehyde by reductive amination: (a) Benzylamine (c) N, N-Dimethylbenzylamine (b) Dibenzylamine (d) N-Benzylpiperidine
-
Show how you could prepare each of the following compounds from cyclopentanone, D2O, and any necessary organic or inorganic reagents. H OH
-
Show how you could prepare each of the following compounds. Use the starting material indicated along with ethyl acetoacetate or diethyl malonate and any necessary inorganic reagents. Assume also...
-
In Exercises 1126, determine whether each equation defines y as a function of x. 4x = y 2
-
Jay Maxey retired a few years ago at age 48, courtesy of the numerous stock options he had been granted while president of e-shops.com, an Internet start-up company. He soon moved to Montana to...
-
Sketch the solid whose volume is given by the sum of the iterated integrals Then write the volume as a single iterated integral in the order dy, dz, dx. Jz/2Jz/2 r6 (12-2)/2 6-y Toz Jz/2 dx dy dz +...
-
Microwaves on sale. The prices of new gadgets often start high and then fall rapidly. The first home microwave oven cost $1300 in 1955. You can now buy a better microwave oven for $100. Find the...
-
Rouse Manufacturing Limited produces and sells one product, a three-foot Canadian flag. During 20X0, the company manufactured and sold 65,000 flags at $27 each. Existing production capacity is 75,000...
-
PLEASE HELP. Your company is planning to borrow $1.75 million on a 5-year, 13%, annual payment, fully amortized term loan. The data has been collected in the Microsoft Excel Online file below. Open...
-
How might you use a reductive amination to synthesize ephedrine, an amino alcohol that is widely used for the treatment of bronchial asthma? CHCHNHCH3 CH3 Ephedrine
-
The amino acid proline is biosynthesized from glutamate semialdehyde by the following transformation, where NADH is the biological reducing agent nicotinamide adenine dinucleotide. What is the likely...
-
Zhang (2008) argues that if a borrower adopts conservative accounting methods this will reduce the risk exposure of the lender and will lead to a reduced interest cost for the borrower. What is the...
-
The process of translating an idea into goods and services that create value or for which clients will pay is called
-
Let f be twice differentiable with f(0) = 6, f(1) = 8, and f'(1) = 7. Evaluate the following integral. [ = 0 0 xf" (x)dx
-
Although the Chen Company's milling machine is old, it is still in relatively good working order and would last for another 10 years. It is inefficient compared to modern standards, though, and so...
-
PART-3: OFFLINE QUESTIONS - Upload files using the submission link. 1. In 2020 Starbucks began a secret project to develop a competing product against the Keurig Single Serve coffee brewer. The...
-
As a leader, what are your highest values? o What's the contribution you want to make as a leader o What makes you distinct as a leader? o Drawing from StrengthsFinder 2.0 what are your strengths as...
-
Derive Sarah's labor supply function given that she has a quasilinear utility function, U = Y0.5 + 2N and her income is Y = wH. What is the slope of her labor supply curve with respect to a change in...
-
CLASS PERIO Solving Linear Equations: Variable on Both Sides Solve each equation. 1) 6r+ 7 = 13 + 7r 3) -7x-3x+2=-8x-8 5)-14 +66+7-26=1+5b 7) n-3n = 14-4n 2) 13-4x=1-x 4)-8-x= x - 4x 6)n+2=-14-n 8)...
-
Identify the reagents necessary to accomplish each of the following transformations. If you are having trouble, the reagents for these transformations appear on page 482, but you should first try to...
-
Identify the reagents you would use to accomplish each of the following transformations: a. b.
-
Identify the reagents you would use to accomplish each of the following transformations: a. b.
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


