The C=O double bond has a dipole moment of about 2.4 D and a bond length of
Question:
The C=O double bond has a dipole moment of about 2.4 D and a bond length of about 1.23 Å.
(a) Calculate the amount of charge separation in this bond.
(b) Use this information to evaluate the relative importance of the following two resonance contributors:
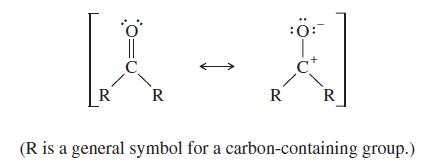
Transcribed Image Text:
:ö:- R R R R (R is a general symbol for a carbon-containing group.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)

Answered By

CHINKY GANGWAR
I had completed my master's in chemistry from university of Lucknow and currently pursuing PhD from the same University. I had worked in bsnv pg college lucknow for 7months as a part time lecturer in department of chemistry.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The iodine mono bromide molecule, IBr, has a bond length of 2.49 and a dipole moment of 1.21 D. (a) Which atom of the molecule is expected to have a negative charge? Explain. (b) Calculate the...
-
The PF3 molecule has a dipole moment of 1.03 D, but BF3 has a dipole moment of zero. How can you explain the difference?
-
Two molecules, each with the general formula AX3, have different dipole moments. Molecule Y has a dipole moment of zero, whereas molecule Z has a nonzero dipole moment. From this information, what...
-
In Exercises simplify the ratio of factorials. (n + 1)! n!
-
Write the chemical reaction whose equilibrium constant is (a) Ka for benzoic acid, C6H5CO2H (b) Kb for benzoate ion, C6H5CO-2 (c) Kb for aniline, C6H5NH2 (d) Ka for anilinium ion, C6H5NH+3
-
What are the main causes of the symptom(s)?
-
a. Write a first-order model relating E(y) to two quantitative independent variables x1 and x2. b. Write a complete second-order model.
-
The national coffee store Farbucks needs to decide in August how many holiday-edition insulated coffee mugs to order. Because the mugs are dated, those that are unsold by January 15 are considered a...
-
A Hedge Fund has a bond exposure of US$ 200 M - Haircut is 70% - Salvage value on this portfolio is
-
Verify the values given in Table 5.3 for NV, NE, and ND for a partial reboiler and a totalcondenser. Ng. Independent Relationships Ny, Total Number of No. Degrees of Freedom Element or Unit Name...
-
Give the relationship between the following pairs of structures. The possible relationships are: same compound cis-trans isomers constitutional isomers (structural isomers) not isomers (different...
-
The N-F bond is more polar than the N-H bond, but NF 3 has a smaller dipole moment than NH 3 . Explain this curious result. NH 3 .... NF 3 = 1.5D = 0.2D
-
When the hydronium ion concentration of a solution is 1 10 -10 M, what is the pH of the solution? Is the solution acidic or basic? When the hydronium ion concentration of a solution is 1 10 -4 M,...
-
Units processed during September for material and conversion. Ask an instructor lock lock lock A 3 A copy Determine the cost per equivalent unit for material and conversion cost combined. copy...
-
12% of all college students volunteer their time. Is the percentage of college students who are volunteers different for students receiving financial aid? Of the 338 randomly selected students who...
-
Mervon Company has two operating departments: mixing and bottling. Mixing has 3 3 0 employees and Bottling has 2 2 0 employees. Indirect factory costs include administrative costs of $ 1 8 2 , 0 0 0...
-
XP Ltd. is a manufacturing company with high stock requirements. Management are currently considering their stockholding policy. The following information is available for one stock item, material...
-
Process Costing: weighted average method Required: make a cost of production report in good form. Cost of Production Report-Weighted Average First Dept- Gem Company applies 100% of materials at the...
-
The right-angle link has a counterclockwise angular velocity of 3 rad /s at the instant represented, and point B has a velocity v B = 2i 0.3j m/s. Determine the velocity of A using vector notation....
-
Vectors are drawn from the center of a regular n-sided polygon in the plane to the vertices of the polygon. Show that the sum of the vectors is zero.
-
Explain why all attempts to isolate trimethyloxonium iodide lead instead to methl iodide and dimethl ether.
-
Give the structure of an intramoleczlar substitution product and an intermolecular substitution product that might be obtained from 4-bromo-l-butanol on treatment with one equivalent of NaOH. Which...
-
Give the structure of an intramoleczlar substitution product and an intermolecular substitution product that might be obtained from 4-bromo-l-butanol on treatment with one equivalent of NaOH. Which...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 0 2 0 2 1 $ 6 4...
-
The adjusted trial balance for Tybalt Construction on December 3 1 of the current year follows. TYBALT CONSTRUCTION Adjusted Trial Balance December 3 1 Number Account Title Debit Credit 1 0 1 Cash $...
-
( US$ millions ) 1 2 / 3 1 / 2 0 1 4 1 2 / 3 1 / 2 0 1 3 1 2 / 3 1 / 2 0 1 2 1 2 / 3 1 / 2 0 1 1 Net income $ 1 4 , 4 3 1 $ 1 2 , 8 5 5 $ 1 0 , 7 7 3 $ 9 , 7 7 2 Depreciation 3 , 5 4 4 2 , 7 0 9 1 ,...

Study smarter with the SolutionInn App


