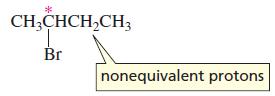
The two hydrogens of a methylene group adjacent to an asymmetric carbon are not equivalent hydrogens because
Question:
The two hydrogens of a methylene group adjacent to an asymmetric carbon are not equivalent hydrogens because they are in different environments due to the asymmetric carbon. (You can verify this statement by examining molecular models.) Applying the N + 1 rule to these two diastereotopic hydrogens (Section 5.16) separately in determining the multiplicity of the signal for the adjacent methyl hydrogens indicates that the signal should be a doublet of doublets. The signal, however, is a triplet. Using a splitting diagram, explain why it is a triplet rather than a doublet of doublets.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





