Question: Three common lab experiments are shown. In each case, describe how the IR spectrum of the product would differ from that of the reactant. Give
Three common lab experiments are shown. In each case, describe how the IR spectrum of the product would differ from that of the reactant. Give approximate frequencies for distinctive peaks in the IR spectrum of the reactant and also that of the product.
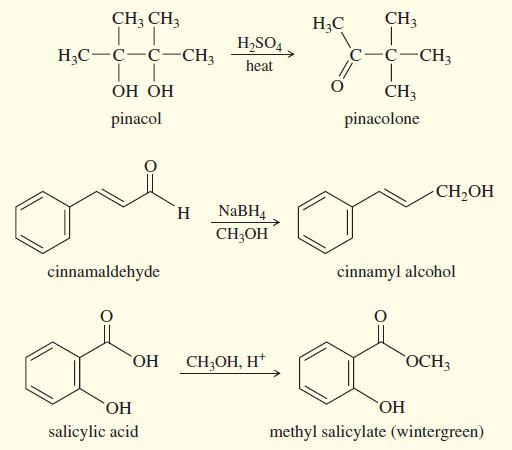
CH3 CH3 H;C CH3 H,SO4. H;C-C-C-CH3 c-C-CH3 heat CH3 pinacol pinacolone CH,OH NABH4 CH;OH H. cinnamaldehyde cinnamyl alcohol HO, CH;OH, H* OCH3 HO, salicylic acid methyl salicylate (wintergreen)
Step by Step Solution
3.46 Rating (159 Votes )
There are 3 Steps involved in it
1 The reactant is an alcohol and the product is a ketone The main differences between t... View full answer

Get step-by-step solutions from verified subject matter experts


