Three IR spectra are shown, corresponding to three of the following compounds. For each spectrum, determine the
Question:
Three IR spectra are shown, corresponding to three of the following compounds. For each spectrum, determine the structure and explain how the peaks in the spectrum correspond to the structure you have chosen.
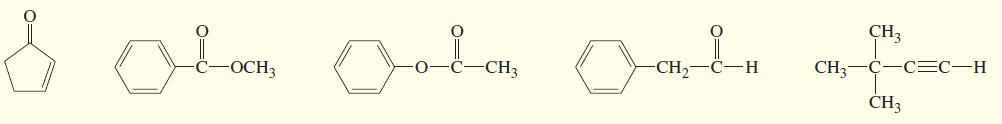
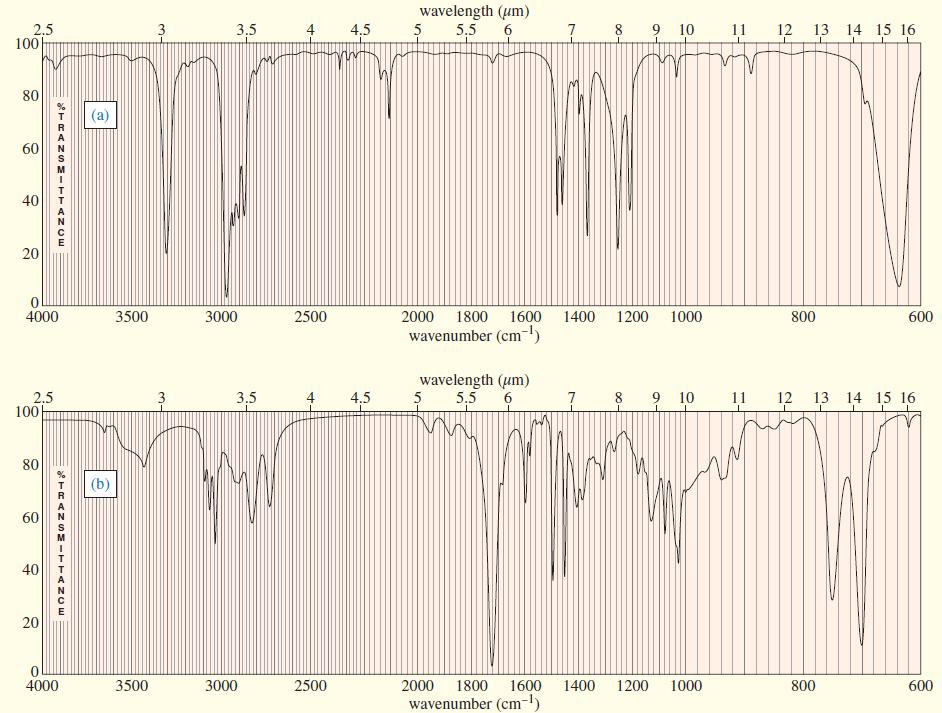
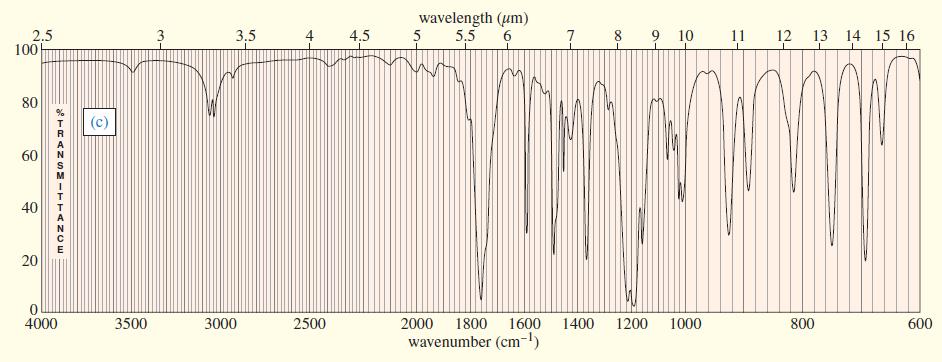
Transcribed Image Text:
CH3 Lon CH CH3 C-C=C-H H. OCH3 CH3 CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)

Answered By

Vaibhav Gupta
I am studying in the number 1 university of India i. e Delhi university as a physicist.
And I am also my school and university topper and I am tutoring around 3 years to many students and they are always satisfied from my tutoring.
I will do my best to provide best explanation with answers.
Thankyou
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Four infrared spectra are shown, corresponding to four of the following compounds. For each spectrum, determine the structure and explain how the peaks in the spectrum correspond to the structure you...
-
The 1H NMR spectrum shown in Figure 14.8 corresponds to one of the following compounds. Which compound is responsible for this spectrum? C CH CH3 CH3 CICH2 CH2C Br2CH CHBr2 6 5 3 2 0 8 (ppm)...
-
The IR spectrum shown in Figure 13.43 is the spectrum of one of the following compounds. Identify the compound. CH2OH COOH CH2NH2 3 2.6 27 28 20 3 12 13 14 5 6 4003360 3400 3200 3000 2800 2800 2400...
-
Compute the given derivatives with the help of formulas (1)(4). (a) (b) d dx (1) - x=e
-
A 0.0450 M solution of HA is 0.60% dissociated. Calculate pKa for this acid.
-
Calculating EFN In Problem 21, suppose the firm wishes to keep its debtequity ratio constant. What is EFN now?
-
A cost that is computed in advance before production starts is (a) Marginal cost (b) Standard cost (c) Predetermined cost (d) Opportunity cost
-
Write a program that produces calendars as output. Your program should have a method that outputs a single months calendar like the one below, given parameters to specify how many days are in the...
-
You went to college with a student named Hank Skully. You remember Hank as a poor student that would always cheat and plagerize on his assignments. Now that Hank is working he can no longer use Chegg...
-
A sample of 1.00 mol perfect gas molecules with Cp.m 7/2R is put through the following cycle: (a) constant-volume heating to twice its initial volume, (b) reversible, adiabatic expansion back to its...
-
Chapter 9 covered a synthesis of alkynes by a double dehydrohalogenation of dihalides. A student tried to convert trans-2,5-dimethylhex-3-ene to 2,5-dimethylhex-3-yne by adding bromine across the...
-
A laboratory student added 1-bromobutane to a flask containing dry ether and magnesium turnings. An exothermic reaction resulted, and the ether boiled vigorously for several minutes. Then she added...
-
Dale is in business as a sole trader. You are presented with the following summarized information relating to his business for the year to 31 October 20X2: Required a. Based on the above information,...
-
How have your organizations performed relative to improving healthcare quality and meeting the required standards (Medicare metrics) for value-based purchasing initiatives?
-
/ Precalculus Algebra Problem. 1: Consider the function f(x)=-5x5 + +-4. How many terms in f(x) are not monomials? Problem. 2: Consider the function f(x)=-3x-4x - 3x + 12. How many terms in f(x) are...
-
D 0
-
What NaCl concentration results when 279 mL of a 0.680 M NaCl solution is mixed with 462 mL of a 0.450 M NaCl solution? concentration: M
-
Use JavaFX's shape's classes from javafx.scene.shape package to complete the following questions (Hint: CANNOT use any Gaphics or Graphics2D classes from java.awt packages): DO not write the whole...
-
The rotation of link AO is controlled by the piston rod of hydraulic cylinder BC, which is elongating at the constant rate s = k for an interval of motion. Write the vector expression for the...
-
Find the reduced echelon form of each of the matrices given in Problems 120. c 1 26 + 4
-
How would the proton NMR spectrum of the com-pound in part (a) change following a D2O shake?
-
Imagine taking the NMR spectrum of a sample of "naked" protons-that is, H+ in the gas phase not chemically bound to anything. In which of the following ranges of chemical shifts would you expect to...
-
The gyromagnetic ratio of the electron is 17.60 106 rad gauss-1 s-1, 658 times greater than that of the proton. What operating frequency would be required to detect the magnetic resonance of an...
-
Construction of consumer price index number for the given goods and services. Item Weight in % Base period price Current period price Food 35 150 145 Fuel 10 25 23 Cloth 20 75 65 Rent 15 30 30 Misc....
-
Gammaro Corporation has found that 80% of its sales in any given month are credit sales, while the remainder are cash sales of the credit sales, Gammaro Corporation has experienced the following...
-
Swifty Company estimates that 2022 sales will be $43,200 in quarter 1,$51,840 in quarter 2 , and $62,640 in quarter 3 , Cost of goods sold is 50% of sales. Management desires to have ending...

Study smarter with the SolutionInn App


