Use Figure 1.14 to order the following molecules according to increasing positive character of the carbon atom:
Question:
Use Figure 1.14 to order the following molecules according to increasing positive character of the carbon atom:![]()
Fig 1.14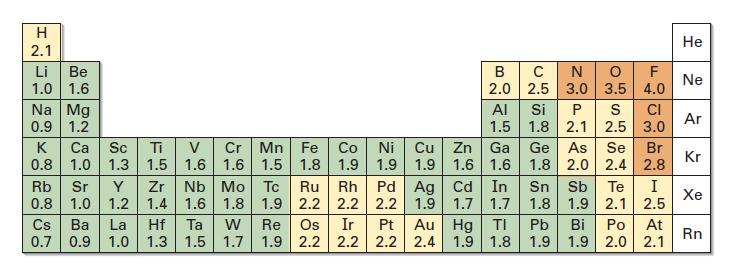
Transcribed Image Text:
CH3F, CH₂OH, CH3Li, CH3I, CH3CH3, CH3NH2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To determine the increasing positive character of the carbon atom in the given molecules we can refe...View the full answer

Answered By

Churchil Mino
I have been a tutor for 2 years and have experience working with students of all ages and abilities. I am comfortable working with students one-on-one or in small groups, and am able to adapt my teaching style to meet the needs of each individual. I am patient and supportive, and my goal is to help my students succeed.
I have a strong background in math and science, and have tutored students in these subjects at all levels, from elementary school to college. I have also helped students prepare for standardized tests such as the SAT and ACT. In addition to academic tutoring, I have also worked as a swim coach and a camp counselor, and have experience working with children with special needs.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Name the following molecules according to the IUPAC system of nomenclature. (a) (b) (c) (d) (e) CH3CH(CH3)CH(CH3)CH(CH3)CH(CH3)2 (f) CH3CH2CHCH3 CH CH H3C CH3 CHCHCH2CH3 EH CHCH,CH.CCH,CH.CH CH,...
-
Name the following molecules according to the IUPAC nomenclature system. (a) (b) (c) (d) (e) (f) CH32 Cl CH3 Br
-
On September 30 of the current year, Silver Fox Corporation files for bankruptcy. At the time, it estimates that the total FMV of its assets is $725,000, whereas the total amount of its outstanding...
-
Under what circumstances may an involuntary petition for relief be filed? Who files this petition?
-
Determine the compressibility factor of steam at 20 MPa, 400oC using (a) The LK chart. (b) The NO chart. (c) The PC model. (d) What-if Scenario: What would the answers be if the steam were saturated...
-
Prepare a statement of cash fl ows using the direct method. LO11
-
1. How did Dansko's founder Peter Kjellerup's Danish heritage affect the development of Dansko's shoe line and its commitment to ethics and social responsibility? 2. Why might employee ownership be a...
-
What is the modified duration for a four-year, semi-annual pay, $1,000 par value, 10.00% coupon bond that is currently priced to yield 8.50%
-
You are discussing your 401(k) with Dan Ervin when he mentions that Sarah Brown, a representative from Bledsoe Financial Services, is visiting East Coast Yachts today. You decide that you should meet...
-
How many valence electrons does each of the following atoms have? (a) Oxygen (b) Magnesium (c) Fluorine
-
The following model is that of aspartame, C 14 H 18 N 2 O 5 , known commercially under many names, including NutraSweet. Only the connections between atoms are shown; multiple bonds are not...
-
Margaret Gilleo is a homeowner in a St. Louis suburb. In December 1990, she placed a 24- by 36-inch sign on her lawn expressing opposition to Operation Desert Storm. She contacted police after her...
-
In Exercises 25-28, construct a data set that has the given statistics. N = 8 2 3
-
Sample SAT scores for eight males and eight females are listed. Males 1010 1170 1410 920 1320 1100 690 1140 Females 1190 1010 1000 1300 1470 1250 840 1060
-
Best Actor 2018: Gary Oldman, Age: 59 Best Supporting Actor 2018: Sam Rockwell, Age: 49 The table shows population statistics for the ages of Best Actor and Best Supporting Actor winners at the...
-
Consider a market dominated by just two airlines, American and United. Each can choose to restrict capacity and charge a high price or expand capacity and charge a low price. If one of the two...
-
Using the product structure for Alpha in Solved Problem 14.1, and the following lead times, quantity on hand, and master production schedule, prepare a net MRP table for Alphas. Data From Problem...
-
Use the following graphs to answer the questions. a. What is the equilibrium quantity of trash collectors hired, and what is the equilibrium wage? b. What is the equilibrium quantity of receptionists...
-
What is a content filter? Where is it placed in the network to gain the best result for the organization?
-
The organoborane used in a Suzuki reaction is prepared by the reaction of catecholborane with an alkene or an alkyne. What hydrocarbon would you use to prepare the organoborane of Problem 35? Problem...
-
The mass spectra of two very stable cycloalkanes both show a molecular ion peak at m/z = 98. One spectrum shows a base peak at m/z = 69, the other shows a base peak at m/z = 83. Identify the...
-
Which of the following compounds has a vibration that is infrared inactive: acetone, 1-butyne, 2-butyne, H 2 , H 2 O, Cl 2 , ethene?
-
Practice Problem 1 The stockholders equity accounts of Bramble Corp. on January 1, 2017, were as follows. Preferred Stock (6%, $100 par noncumulative, 4,400 shares authorized) $264,000 Common Stock...
-
JVCU Which of the following is considered cash for financial reporting purposes? 1 JVCU Which of the following is considered cash for financial reporting purposes? 1
-
Required information The Foundational 15 [LO8-2, LO8-3, LO8-4, LO8-5, LO8-7, LO8-9, L08-10) (The following information applies to the questions displayed below.) Morganton Company makes one product...

Study smarter with the SolutionInn App


