What alcohols might the following alkenes be made from? (a) CH3 CH3 (b) CH3CHCH=CHCHCHCH3
Question:
What alcohols might the following alkenes be made from?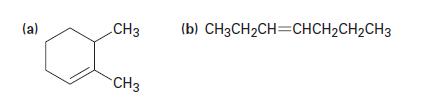
Transcribed Image Text:
(a) CH3 CH3 (b) CH3CH₂CH=CHCH₂CH₂CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To determine the alcohols that the given alkenes might be made from we need to identify the possible ...View the full answer

Answered By

Surendar Kumaradevan
I have worked with both teachers and students to offer specialized help with everything from grammar and vocabulary to challenging problem-solving in a range of academic disciplines. For each student's specific needs, I can offer explanations, examples, and practice tasks that will help them better understand complex ideas and develop their skills.
I employ a range of techniques and resources in my engaged, interesting tutoring sessions to keep students motivated and on task. I have the tools necessary to offer students the support and direction they require in order to achieve, whether they need assistance with their homework, test preparation, or simply want to hone their skills in a particular subject area.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What alkenes might be used to prepare the following alcohols by hydroboration/oxidation? (a) CH3 (b) CHH (c) .CH- CHCHCH2CH2OH
-
What alkenes might the following alcohols have been prepared from? (b) (a) CH3CCH2CH2CH2CH3 C
-
What alkyl halides might the following alkenes have been madefrom? (b) C CH (a) (a) CH CHH2H2%3DCH2 "CH
-
In Problems 25 54, solve each system. Use any method you wish. 2xxy + y = 8 xy = 4
-
A firm has $ 1.5 million in sales, a Lerner index of 0.57, and a marginal cost of $ 50, and competes against 800 other firms in its relevant market. a. What price does this firm charge its customers?...
-
Discuss the key components of a comprehensive risk management strategy withinthe context of supplier management. Provide examples of potential risks and theircorresponding mitigation strategies. ( 1...
-
Brain size and intelligence. For centuries people have associated intelligence with brain size. A recent study used magnetic resonance imaging to measure the brain size of several individuals. The IQ...
-
A large retailer reported revenue of $1,665,000. The companys gross profit percentage was 44 percent. What amount of cost of goods sold did the company report?
-
3031 Which of the following statements is true regarding the allowance method? Awrite-off at an uncollectible account decreases tetained camnings It is not accepted under GAAP An estimate of bad debt...
-
What effect would the following changes have on the rate of the S N 1 reaction of tert-butyl alcohol with HBr? (a) The HBr concentration is tripled. (b) The HBr concentration is halved, and the...
-
What product would you expect to obtain from the S N 2 reaction of (S)-2 bromohexane with sodium acetate, CH 3 CO 2 Na? Show the stereochemistry of both product and reactant.
-
What are the top three digital ethics issues at the moment?
-
Sample grade point averages for ten male students and ten female students are listed. Males 2.4 3.7 3.8 3.9 Females 2.8 3.7 2.1 3.9 2.8 2.6 3.6 3.3 4.0 1.9 3.6 4.0 2.0 3.9 3.7 2.3
-
Fill in the columns in the following table. What quantity should a profit-maximizing firm produce? Verify your answer with marginal reasoning. 9 0 1 2 3 st 4 5 6 TFC $5 5 5 5 5 5 5 TVC $0 3 5 9 16 25...
-
Perform the experiments in Problems 48-51, tally your results, and calculate the probabilities (to the nearest hundredth). Flip three coins simultaneously 100 times, and note the results. The...
-
The following information is available for Spring Inc. and Winter Inc. at December 31, 2011: Required a. What is the accounts receivable turnover for each of the companies for 2011? b. What is the...
-
Margin of error = 0.5 g, standard deviation = 8.7 g
-
Rewrite the following if-else chain using a switch statement If (lettergrade=='A') printf("The numerical grade is between 90 and 100 "); else if((lettergrade=='B') printf("The numerical grade is...
-
Consider the following cash flows in Table P5.5. (a) Calculate the payback period for each project. (b) Determine whether it is meaningful to calculate a payback period for project D. (c) Assuming...
-
Identify whether each of the following reagents would be a strong nucleophile or a weak nucleophile, and also indicate whether it would be a strong base or a weak base: a.
-
We have seen that NaH is a strong base but a weak nucleophile. In contrast, lithium aluminum hydride (LAH) is a reagent that can serve as a source of nucleophilic hydride ion: In this case, LAH...
-
a) NaOH is a strong nucleophile and strong base. The substrate in this case is primary. Therefore, we expect S N 2 (giving the major product) and E2 (giving the minor product). b) NaSH is a strong...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


