What is the index of hydrogen deficiency (IHD) (degree of unsaturation) for each of the following compounds?
Question:
What is the index of hydrogen deficiency (IHD) (degree of unsaturation) for each of the following compounds?
a.
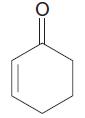
b. C6H8Br4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
Index of hydrogen deficiency IHD degree of unsaturation Method This method is most commonly used Acc...View the full answer

Answered By

Harshal Saraf
I am Post Graduate in Organic Chemistry & qualified CSIR-NET-JRF & SET exam. In addition to this I have also qualified GATE-2012. I have five years teaching experience at senior level in Pratap College, Amalner.
Academic teaching record and teaching Interest:-
[1] Qualified CSIR-NET-JRF exam in June 2012 with UGC-JRF
[2] Qualified SET/ UGC-SLET exam (State Government) in Dec 2013 and Sept 2015
[3] Qualified GATE exam (MHRD-IIT) in February 2012
[4] Total Teaching experience :7 years (UG and PG Level)
[5] Book Publication: 2 (Topic wise complete solution of the CSIR-NET-JRF and GATE Exam,
Himalaya Publication.)
[6] Taught Organic Reaction Mechanism, Photochemistry, Stereochemistry and Natural product chemistry
to M.Sc. Organic class
[7] Taught Non-transition, Transition metal chemistry, electronic spectroscopy, point group and group
theory to M.Sc. Inorganic class
[8] Taught electrochemistry, chemical kinetics to B. Sc class
[9] Taught Quantum Chemistry, Electrochemistry, Molecular spectroscopy and statistical thermodynamics
to M. Sc. Physical class.
[10] Given lecture series for the Masters students for the preparation of the NET, SET & GATE exams.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118133576
11th edition
Authors: Graham Solomons, Craig Fryhle, Scott Snyder
Question Posted:
Students also viewed these Sciences questions
-
What is the index of hydrogen deficiency for (a) C 7 H 10 O 2 (b) C 5 H 4 N 4 ?
-
(a) What is the index of hydrogen deficiency of 2-hexene? (b) Of methylcyclopentane? (c) Does the index of hydrogen deficiency reveal anything about the location of the double bond in the chain? (d)...
-
Squalene, an important intermediate in the biosynthesis of steroids, has the molecular formula C30H50 and has no triple bonds. (a) What is the index of hydrogen deficiency of squalene? (b) Squalene...
-
In Exercises approximate the definite integral using the Trapezoidal Rule and Simpson's Rule with n = 4. Compare these results with the approximation of the integral using a graphing utility. 3.1 J3...
-
At what point in the titration of a weak base with a strong acid is the maximum buffer capacity reached? This is the point at which a given small addition of acid causes the least pH change.
-
an unexpected change in a variable Match each term with its correct definition by placing the appropriate letter next to the corresponding number. A. Phillips curve E. shock B. reservation wage F....
-
Whales entangled in fishing gear. Refer to the Marine Mammal Science (Apr. 2010) study of whales entangled in fishing gear, Exercise 12.87, (p. 731). Now consider a model for the length (y) of an...
-
Assume that today is September 12. You have been asked to help a British client who is scheduled to pay 1,500,000 on December 12, 91 days in the future. Assume that your client can borrow and lend...
-
E-Tech Initiatives Limited plans to issue $550,000, 10-year, 3 percent bonds. Interest is payable annually on December 31. All of the bonds will be issued on January 1, 2019. Show how the bonds would...
-
Keep the same weekly sales distribution for Best Car as in Example but assume that the price of cars is distributed as follows: Sales Price (price/car) Relative Frequency (probability) $18,000...
-
Without consulting tables, arrange the following compounds in order of decreasing acidity: Pentane 1-Pentene 1-Pentyne 1-Pentanol
-
Give the structure and name of the product that would be obtained from the ionic addition of IBr to propene.
-
In a recent newspaper release, the president of Heller Company asserted that something has to be done about depreciation. The president said, "Depreciation does not come close to accumulating the...
-
(b) A cylindrical storage tank with base area 90 m is being filled with water through an entry duct with cross-section area 250 cm, as shown in Figure 3. Concurrently, water is being extracted from...
-
Po A cylinder/piston arrangement contains 5 kg of water at 100 C with x= 20%. Initially the piston of mass m, 75 kg rests on a set of stops (see figure). The outside pressure is 100 kPa, and the area...
-
TABLE 2 Present Value of an Annuity of $1 n 123456 8% 9% 0.925926 0.917431 4 7 8 9 10 11 12 13 14 15 16 17 11.652296 10.837770 10.105895 9.446649 12.165669 11.274066 10.477260 9.763223 18 19...
-
There are 4 suits (heart, diamond, clover, and spade) in a 52-card deck, and each suit has 13 cards. Suppose your experiment is to draw one card from a deck and observe what suit it is. Express the...
-
Write isotopic symbol of zirconium and how many neutrons are present in one atom of this isotope
-
Let y 1 , y 2 , . . . , y n be a random sample of n observations from a Poisson distribution with probability function a. Find the maximum likelihood estimator of . b. Is the maximum likelihood...
-
Using the information in P11-2B, compute the overhead controllable variance and the overhead volume variance. Data From Problem 11-2B: Huang Company uses a standard cost accounting system to account...
-
When peptides containing the Asn-Gly sequence, such as H in the equation given in Fig. P26.69, are stored in aqueous solution at neutral or slightly basic solution, ammonia is liberated and a...
-
When peptides containing the Asn-Gly sequence, such as H in the equation given in Fig. P26.69, are stored in aqueous solution at neutral or slightly basic solution, ammonia is liberated and a...
-
(a) Point out the ionizable groups of the amino acid tyrosine (Table 26.1). (b) What is the net charge on tyrosine at pH 6? How do you know? (c) Draw the structure of the major form(s) of tyrosine...
-
Show that the convexity for a zero coupon bond with m payments per year is (m) n(n + -)(1+ m m
-
Abdul Canarte , a Central Bank economist, noticed that the total group purchasing basket of goods (CPI) has gone from $149,740.00 to $344,460.00 in 8 years. With monthly compounding, what is the...
-
ABC Corporation expects sales next year to be $50,000,000. Inventory and accounts receivable (combined) will increase $8,000,000 to accommodate this sales level. The company has a profit margin of 6...

Study smarter with the SolutionInn App


