Where in the infrared spectrum would you expect each of the following compounds to absorb? (a) (b)
Question:
Where in the infrared spectrum would you expect each of the following compounds to absorb?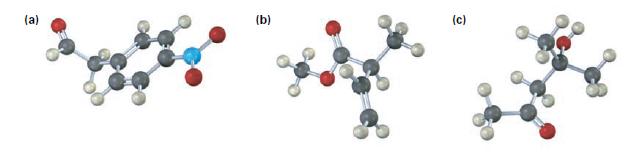
Transcribed Image Text:
(a) (b) (C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
Based on the structures of the compounds in the image I would expect them to absorb in the following ...View the full answer

Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Where would you expect each of the following compounds to absorb in the IR spectrum? (a) 4-Penten-2-one (b) 3-Penten-2-one (c) 2, 2-Dimethylcyclopentanone (d) m-Chloro benzaldehyde (e)...
-
Where in the IR spectrum would you expect each of the following molecules toabsorb? (a) (c) (b)
-
All of the following compounds absorb infrared radiation between 1600 and In each case, 1. Show which bonds absorb in this region. 2. Predict the approximate absorption frequencies. 3. Predict which...
-
In Exercises 912, use the given conditions to write an equation for each line in point-slope form and general form Passing through (4, -7) and perpendicular to the line whose equation is x - 2y - 3 =...
-
The following data pertain to three divisions of Calrisian Enterprises. The companys required rate of return on invested capital is 8 percent. Required: Fill in the blanksabove. Division I Division...
-
Verify the given moment(s) of inertia and find x and y. Assume that each lamina has a density of = 1 gram per square centimeter. (These regions are common shapes used in engineering.) Quarter circle...
-
What attributes should this small business use to evaluate Staples as vendor? What about Lendio? Using those attributes for Staples and Lendio, perform two separate vendor analyses. STAPLES: THE...
-
Assume that you recently received your MBA and now work as assistant to the CFO of a relatively large corporation. Your boss has asked you to prepare a financial forecast for the coming year, using...
-
It will cost $3 million to develop the show. There are eight performances per week (on Saturday there is a matinee) and we expect the show to run for 52 weeks. It costs $800 to open up the theatre...
-
In light of your answer to Problem 12.47, which would you expect to be more basic, aniline or p-methoxyaniline? Explain. Problem 12.47 How can you explain the observation that p-nitroaniline is less...
-
How can you explain the observation that p-nitroaniline is less basic than aniline by a factor of 40,000?
-
Convert 25p rad (a) To revolutions. (b) To degrees.
-
A pistoncylinder device contains 0.85 kg of refrigerant-134a at 210 oC. The piston that is free to move has a mass of 12 kg and a diameter of 25 cm. The local atmospheric pressure is 88 kPa. Now,...
-
3.3. Using the BEMT, show the effect of increasing linear twist on the variations in inflow, thrust, induced power, profile power, and lift coefficient across the span of a rotor with four blades of...
-
By uploading this work, I attest that the work contained herein is solely my own, that I only used the given equation sheet as a reference, and that I have not received any information from anyone...
-
Demand for patient surgery at Washington General Hospital has increased steadily in the past few years, as seen in the following table: ...
-
Explain product analysis
-
In the short run, a firm cannot vary its capital, K = 2, but it can vary its labor, L. It produces output q. Explain why the firm will or will not experience diminishing marginal returns to labor in...
-
Jax Incorporated reports the following data for its only product. The company had no beginning finished goods inventory and it uses absorption costing. $ 57.30 per unit $ 10.30 per unit $ 7.80 per...
-
Consider the structure of the following compound: (a) When this compound is treated with bromine under conditions that favor monobromination, two stereoisomeric products are obtained. Draw them, and...
-
Name one element that you would expect to exhibit bonding properties similar to boron. Explain?
-
Nicotine is an addictive substance found in tobacco. Identify the hybridization state and geometry of each of the nitrogen atoms in nicotine: C-H N. C- H. Nicotine z: I I-O
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...
-
Which of the following concerning short-term financing methods is NOT CORRECT? Short-term bank loans typically do not require assets as collateral. Firms generally have little control over the level...
-
Kingbird Corporation is preparing its December 31, 2017, balance sheet. The following items may be reported as either a current or long-term liability. 1. On December 15, 2017, Kingbird declared a...

Study smarter with the SolutionInn App


