Which molecules in Practice Problem 1.14 form sets of constitutional isomers? Problem 1.14 (a) (CH 3 )
Question:
Which molecules in Practice Problem 1.14 form sets of constitutional isomers?
Problem 1.14
(a) (CH3)2CHCH2CH3
(b) (CH3)2CHCH2CH2OH
(c) (CH3)2C=CHCH2CH3
(d) CH3CH2CH2CH2CH3
(e) CH3CH2CH(OH)CH2CH3
(f) CH2=C(CH2CH3)2
(g)
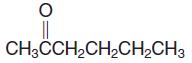
(h) CH3CHClCH2CH(CH3)2
Transcribed Image Text:
CH;ČCH,CH2CH2CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (16 reviews)
a and d Chain isomer b and e Posi...View the full answer

Answered By

Nidhi Singh Hada
I am faculty of Chemistry. I have 14 experience of teaching chemistry. I worked in various organisations. It gave me a lot off experience. I have equal command on all the chemistry portion. I teach organic, inorganic and Physical chemistry.
My own method of teaching and solving the question in different methods made the subject easier for students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118133576
11th edition
Authors: Graham Solomons, Craig Fryhle, Scott Snyder
Question Posted:
Students also viewed these Sciences questions
-
Write each of the following condensed structural formulas as a bond-line formula: (a) (CH3)2CHCH2CH3 (b) (CH3)2CHCH2CH2OH (c) (CH3)2C==CHCH2CH3 (d) CH3CH2CH2CH2CH3 (e) CH3CH2CH(OH)CH2CH3 (f) CH2...
-
For each compound in Problem 15, determine how many constitutional isomers can form upon monohalogenation. Problem 15 (a) CH3CH2CH2CH3 (b) CH3CH2CH2CH2CH3 (c) (d) CH3 ,
-
Predict which compound in each pair has the higher boiling point. Explain your prediction. (a) CH3CH2OCH3 or CH3CH1OH2CH3 (b) CH3CH2CH2CH3 or CH3CH2CH2CH2CH3 (c) CH3CH2CH2CH2CH3 or (CH3)2CHCH2CH3 (d)...
-
Visit www.guidestar.org and obtain the Form 990 for a local not-for-profit organization. a. Examine Part VIII of the 990 to determine gross receipts of the organization. b. Examine Part IX of the...
-
Prepare a family of graphs for the titration of 50.0 mL of 0.0200 M H2A with 0.100 M NaOH. Consider the following cases: (a) pK1 = 4.00, pK2 8.00; (b) pK1 = 4.00, pK2 = 6.00; (c) pK1 = 4.00, pK2 = 5.
-
Calculating NPV For the cash flows in the previous problem, what is the NPV at a discount rate of zero per cent? What if the discount rate is 5 per cent? If it is 15 per cent? If it is 25 per cent?
-
Dating and disclosure. Refer to the Journal of Adolescence (Apr. 2010) study of adolescents disclosure of their dating and romantic relationships, Exercise 8.43 (p. 419). Data collected for a sample...
-
The county replaced its old office building with a new structure. Rather than destroy the old office building, the county decided to convert the old building and use it as a storage facility. Why...
-
i only need the last picture solved. The rest is just information to help you solve it. input sheet: master budget sheet problem i need solved 1 Master Budget Okay Co. 2 Input Section 3 4 6 5 Sales...
-
The Budvar Company sells parts to a foreign customer on December 1, Year 1, with payment of 20,000 crowns to be received on March 1, Year 2. Budvar enters into a forward contract on December 1, Year...
-
There are actually three constitutional isomers with the molecular formula C 3 H 8 O. We have seen two of them in propyl alcohol and isopropyl alcohol. Write a dash formula for the third isomer.
-
What do the bond angles of ammonia suggest about the hybridization state of the nitrogen atom of ammonia?
-
MalMax purchased a depreciable asset. What would be the difference in total assets at the end of the first year if MalMax chooses straight-line depreciation versus doubledeclining-balance...
-
(Reference: A Closer Look on Cost Accounting, De Jesus, 2019) Paulo Corporation had the following account balances as of August 1, 2020: Raw materials inventory (direct and indirect) Work in Process...
-
Use the given data values (a sample of female arm circumferences in centimeters) to identify the corresponding z scores that are used for a normal quantile plot, then identify the coordinates of each...
-
1. What stakeholders other than customers, suppliers, and partners should be considered when conceiving ways to monetize data? 2. What would be one strategy to maximize "reliability" attribute in...
-
What important guidance does little's law offer healthcare leadership regarding the planning and utilization of resources
-
Semester Two Practice Examinations, 2022 STAT2201 Question 2. [10 marks] A study investigated the effect of playing computer games on heart rate. Twenty eight individuals were recruited into the...
-
The 32.2-lb wheel is released from rest and rolls on its hubs without slipping. Calculate the speed v of the center O of the wheel after it has moved a distance x = 10 ft down the incline. The radius...
-
A liquid flows upward through a valve situated in a vertical pipe. Calculate the differential pressure (kPa) between points A and B. The mean velocity of the flow is 4.1 m/s. The specific gravity of...
-
(a) Give the structure of cocaine (Fig. 23.4) as it would exist in 1 M aqueous HCI solution. (b) What products would form if cocaine were treated with an excess of aqueous NaOH and heat? (c) What...
-
(a) Give the structure of cocaine (Fig. 23.4) as it would exist in 1 M aqueous HCI solution. (b) What products would form if cocaine were treated with an excess of aqueous NaOH and heat? (c) What...
-
How would the basicity of trifluralin, a widely used herbicide, compare with that of N,N-diethylaniline: much greater, about the same, or much less? Explain Et Et O,N NO CF trifluralin
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


