Which of the following compounds are chiral? Label all chirality centers. (a) (d) H3C CH3 | |
Question:
Which of the following compounds are chiral? Label all chirality centers.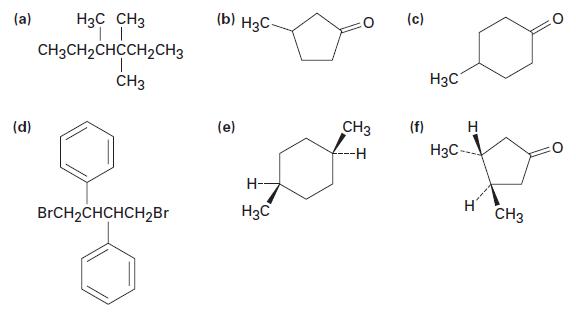
Transcribed Image Text:
(a) (d) H3C CH3 | | CH3CH₂CHCCH₂CH3 CH3 BrCH₂CHCHCH₂Br (b) H3C (e) Н- H3C 0 CH3 (c) (f) H3C Н H3C-- H CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
a The given compound has a chirality center and no plane of ...View the full answer

Answered By

Samee Ullah
Algebra, Linear algebra, calculus, accounting, marketing, statistics, programming, real estate, writing, human resource management, business communication, Engineering: civil, chemical, electrical, mechanical, aerospace, building
Linguistics: sociolinguistics, applied linguistics, music, social sciences, biology, chemistry: all types, Thermodynamics, mechanics, modern physics, quantum physics, metaphysics, biology.
Feel free to contact us for all these subjects,; for quality, and best responses. Thankyou
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following compounds are chiral? Draw each compound in its most symmetric conformation, star (*) any asymmetric carbon atoms, and draw any mirror planes. Label any meso compounds. You may...
-
Which of the following compounds are aromatic? a. b. c. Cycloheptatrienyl cation d. e. f. g. Cyclononatetraenyl anion h. CH2=CHCH=CHCH=CH2
-
Which of the following compounds are chiral? (a) 2-Methylheptane (b) 3-Methylheptane (c) 4-Methylheptane (d) 1,1-Dibromopropane (e) 1,2-Dibromopropane (f) 1,3-Dibromopropane (g) Ethene, H2C=CH2 (h)...
-
A manufacturing company reports the following information for the month of May. Note: Assume all raw materials were used as direct materials. Activities for May Advertising expense Raw materials...
-
The following sale related transactions for Budget Decor, Inc., occurred during the month of April. Apr 3 Sold $3,200 (cost $1,400) of merchandise on account to B. Levin. Terms, 1/15, n/45, FOB...
-
Birthweights of full-term newborns in the United States are approximately normally distributed with a mean of about 3,500 grams and a standard deviation of about 450 grams. We will assume this is...
-
Continual improvement: as a permanent organizational objective, recognizing and acting on the fact that in all cases further improvement is possible? LO.1
-
For a recent year, OfficeMax and Staples are two companies competing in the retail office supply business. OfficeMax had a net income of $71,155,000, while Staples had a net income of $881,948,000....
-
Government regulations designed to reduce the moral hazard problem include O state licensing restrictions. O light sentences for those who commit the fraud of hiding and stealing profits. O state...
-
The following structure represents a carbocation. Draw two resonance structures, indicating the positions of the double bonds.
-
Synthesize the following substances from benzene: (a) o-Bromotoluene (b) 2-Bromo-1,4 dimethylbenzene
-
We Scream For Ice Cream sells ice cream in three flavors: Chocolate, Strawberry, and Vanilla. It sold 28,000 gallons last year, but it is still losing money. For every five gallons of ice cream sold,...
-
IFRS Financial Statements Thomson Reuters is a global information company created by the 2008 merger of the Thomson Corporation, a Canadian company, with the Reuters Company, a United Kingdom-based...
-
Burgess Services Co. experienced the following events in 2011: 1. Provided services on account. 2. Collected cash for accounts receivable. 3. Attempted to collect an account and, when unsuccessful,...
-
In Exercises 13 and 14, use the box-and-whisker plot to identify the five-number summary. 0 2 5 8 10 ++ ++ 0 1 2 3 4 5 6 7 8 9 10 11
-
In a test of the effect of dampness on electrical connections, 80 electrical connections were tested under damp conditions and 130 were tested under dry conditions. Twenty of the damp connections...
-
Zelta Ltd. is a medium-size company involved in providing a range of specialized products and services for the aerospace industry. Just over a year ago, external consultants undertook a major review...
-
A certain crane can provide a maximum lifting force of 25000 N. It hoists a 2000 kg load starting at ground level by applying the maximum force for a 2 second interval; then, it applies just...
-
Solve each equation or inequality. |6x8-4 = 0
-
In one industrial synthesis of ethanol, ethene is first dissolved in 95% sulfuric acid. In a second step water is added and the mixture is heated. Outline the reactions involved.
-
How many signals would you expect to obtain in the 1 H NMR spectrum of undecadeuteriocyclohexane at room temperature?
-
How many signals would you expect to obtain in the 1 H NMR spectrum of undecadeuteriocyclohexane at room temperature?
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


