Which of the following structures represents a cis isomer? CH3 CH, CH3 CH3 -CH, CH3 CH3 CH3
Question:
Which of the following structures represents a cis isomer?
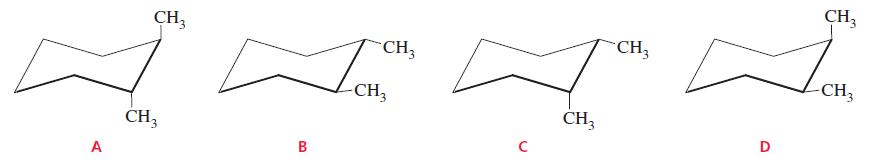
Transcribed Image Text:
CH3 CH, CH3 CH3 -CH, CH3 CH3 CH3 D B A
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following structures has the smallest heat of combustion? (a) (b) (c) (d) CH3 CH3 CH3 CH3 CH3
-
Which of the following diagrams most likely represents an ionic compound, and which represents a molecular one? Explain your choice. (i) (ii)
-
Which of the following compounds can exist as cis-trans isomers? Draw their structures. a. Pentene b. 3-heptene c. 2-methyl-2-pentene d. 2-hexene
-
Summarize the extract given below? When Paul Farmer graduated from Duke University at 22, he was unsure whether he wanted to be an anthropologist or a doctor. So he went to Haiti. As a student, Paul...
-
(a) To detect the drug ibuprofen by liquid chromatography/ mass spectrometry, would you choose the positive or negative ion mode for the spectrometer? Would you choose acidic or neutral...
-
Think again of the worst co-worker youve ever hadone who did some of the things listed in Table 1-1. Think of what that co-workers boss did (or didnt do) to try to improve his or her behaviour. What...
-
Spreading rate of spilled liquid. Refer to the Chemical Engineering Progress (Jan. 2005) study of the rate at which a spilled volatile liquid will spread across a surface, presented in Exercise 2.168...
-
On September 1, Cool Salad Dressings creates a petty cash fund with an imprest balance of $250. During September, Michael Martell, the fund custodian, signs the following petty cash tickets: On...
-
In profit and loss statement of a paper mill company revenue from stock-in-trade is not mentioned separately. But purchases of stock-in-trade is mentioned in expenses section. Only revenue from...
-
Ellipses Corp is a small business that operates in Herndon, VA. The company is located at10 Period Lane, Herndon, VA 20170. Its federal Employer Identification Number is 77-7777777, and its...
-
Ansaid and Motrin belong to the group of drugs known as nonsteroidal anti-inflammatory drugs (NSAIDs). Both are only slightly soluble in water, but one is a little more soluble than the other....
-
Determine the degree of unsaturation for the hydrocarbons with the following molecular formulas: a. C 10 H 16 b. C 20 H 34 c. C 8 H 16 d. C 12 H 20 e. C 40 H 56
-
Let F 1 and F 2 be d.f.s, and let G be their convolution, G = F 1 * F 2 . Then: (i) If F 1 is absolutely continuous with respect to Lebesgue measure with p.d.f. p 1 , it follows that G is absolutely...
-
Management is what tradition used to call a liberal art: "liberal" because it deals with the fundamentals of knowledge, self-knowledge, wisdom, and leadership; "art" because it is a practice and...
-
Draft a five hundred and twenty five- to seven hundred-word internal communication planthat appropriately details your proposed solution to the internal team at CVS PHARMACY. In your communication...
-
Christopher Awnings was founded by Christopher Aminim in the early days of the retirement boom in the Okanagan to build and install custom retractable awnings for retirees to keep the sun out of the...
-
Leaders are responsible for making decisions that have long-term ramifications; thus, making the appropriate decisions can be stressful and leaders' decisions may vary. They often enhance employee...
-
Employee longevity A large insurance company has developed a model to identify the factors associated with employee turnover. The dependent variable is number of years an employee stays with the...
-
Prove Property 10. 10 If m < f(x, y) < M for all (x, y) in D, then A(D) f(x, y) dA < M A(D)
-
Use Stokes' Theorem to evaluate f(y+sin x) dx+(z+cos y) dy+rdz, where C is the rve r(t) = (sint, cost, sin 2t), t = [0, 2].
-
(1) 2,4 - dimethylbenzylamine (2) 2,4,6-trmethylaniline (3) NJV-dimethyl-p-methylaniline (4) 3,5-dimethyl-N-methylaniline (5) 4-ethyl-2,6-dimethylaniline 2.07 (6H, s), 2.16 (3H, s), 3.19
-
Outline a sequence of reactions that would bring about the conversion of aniline into each of the following compounds. (a) Benzyl alcohol (b) N-phenyl-2-butanamine
-
When p-aminophenol reacts with one molar equivalent of acetic anhydride, a compound acetaminophen (A, C8H9NO2) is formed that dissolves in dilute NaOH. When A is treated with one equivalent of NaOH...
-
You are evaluating a new project for the firm you work for, a publicly listed firm. The firm typically finances new projects using the same mix of financing as in its capital structure, but this...
-
state, "The subscription price during a rights offering is normally r; lower ; lower r; higher er; higher than the rights-on price and
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...

Study smarter with the SolutionInn App



