Give the structure of the product formed when (a) 3-methylhexanoic acid is heated with a large excess
Question:
Give the structure of the product formed when
(a) 3-methylhexanoic acid is heated with a large excess of ethanol (as solvent) with a sulfuric acid catalyst.
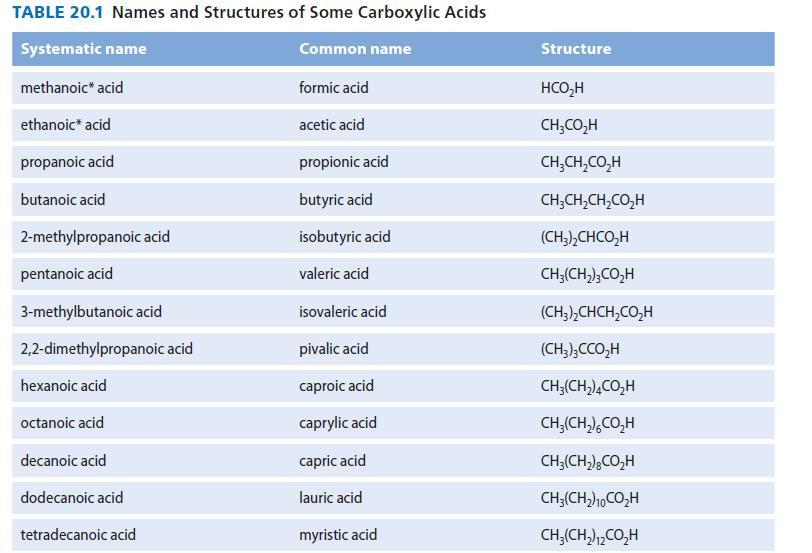
(b) Adipic acid (Table 20.1) is heated in a large excess of 1-propanol (as solvent) with a sulfuric acid catalyst.
Transcribed Image Text:
TABLE 20.1 Names and Structures of Some Carboxylic Acids Systematic name Common name methanoic acid ethanoic acid propanoic acid butanoic acid 2-methylpropanoic acid pentanoic acid 3-methylbutanoic acid 2,2-dimethylpropanoic acid hexanoic acid octanoic acid decanoic acid dodecanoic acid tetradecanoic acid formic acid acetic acid propionic acid butyric acid isobutyric acid valeric acid isovaleric acid pivalic acid caproic acid caprylic acid capric acid lauric acid myristic acid Structure HCO₂H CH,CO,H CH,CH,CO;H CH,CH,CH,CO,H (CH3),CHCO,H CH₂(CH₂)3CO₂H (CH3),CHCH,CO,H (CH3)3CCO₂H CH₂(CH₂)4CO₂H CHJ(CH,)%CO,H CH3(CH2)gCO;H CH,(CH2)CO,H CH₂(CH₂)2CO₂H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a b i CH3CHCHCHCHCOH CH3 HSO4 cat CH3CHOH excess CH3...View the full answer

Answered By

Sarfraz gull
have strong entrepreneurial and analytical skills which ensure quality tutoring and mentoring in your international business and management disciplines. Over last 3 years, I have expertise in the areas of Financial Planning, Business Management, Accounting, Finance, Corporate Finance, International Business, Human Resource Management, Entrepreneurship, Marketing, E-commerce, Social Media Marketing, and Supply Chain Management.
Over the years, I have been working as a business tutor and mentor for more than 3 years. Apart from tutoring online I have rich experience of working in multinational. I have worked on business management to project management.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the structure of the product formed when 3-methylhexanoic acid is heated with a large excess of ethanol (as solvent) with a sulfuric acid catalyst.
-
Give the structure of the expected product formed when benzylamine reacts with each of the following reagents: (a) Hydrogen bromide (b) Sulfuric acid (c) Acetic acid (d) Acetyl chloride (e) Acetic...
-
Give the structure of the expected product formed when benzylamine reacts with each of the following reagents: (a) Hydrogen bromide (b) Sulfuric acid (c) Acetic acid (d) Acetyl chloride (e) Acetic...
-
What do you think people would say about Corrie from the few quotes we have from her book? What was her personality like? Do you think she handled her incarceration differently than Elie Wiesel?...
-
The trial balance of Racer Internet, Inc., at March 31, 2012, follows: Adjusting data at March 31, 2012: a. Unearned service revenue still unearned, $500. b. Prepaid rent still in force, $2,000. c....
-
The income statement of Fezzik's Shoe Repair is as follows: On April 1, the Owner's Capital account had a balance of $12,900. During April, Fezzik withdrew $3,000 cash for personal use. Prepare...
-
Heights of young men. The distribution of heights of young men is approximately Normal with mean 70 inches and standard deviation 2.5 inches. Between which heights do the middle 95% of men fall?...
-
Suppose in the previous problem that HISC always needs a conveyor belt system; when one wears out, it must be replaced. Which system should the firm choose now? In the previous problem, Hagar...
-
If the expected rate of return on the market portfolio is 12% and T-bills yield 4%, what must be the beta of a stock that investors expect to return 9%? (Round your answer to 4 decimal places.) Beta...
-
At a given concentration of acetic acid, in which solvent would you expect the amount of acetic acid dimer to be greater: CCl 4 or water? Explain.
-
Give the structure of each of the following compounds. (a) g-hydroxybutyric acid (b) b,b-dichloropropionic acid (c) (Z)-3-hexenoic acid (d) 4-methylhexanoic acid (e) 1,4-cyclohexanedicarboxylic acid...
-
Road Gear manufactures accessories for road and mountain bicycles. The market for cycling accessories is very competitive, so Road Gear uses a standard costing system to control costs. The day-to-day...
-
1 . Journalize the following transactions: ( a ) Issued 1 , 0 0 0 shares of $ 1 0 par common stock at $ 5 9 for cash. ( b ) Issued 1 , 4 0 0 shares of $ 1 0 par common stock in exchange for equipment...
-
Using alpha .05, determine if moving to a larger enclosure decreased tiger anxiety levels. You should first calculate the difference (After - Before) Tiger Before Anthony 45 45 Banthony 56 After 38...
-
Cyclohexane (C 6 H 12 ) is produced by mixing Benzene and hydrogen. A process including a reactor, separator, and recycle stream is used to produce Cyclohexane. The fresh feed contains 260L/min C 6 H...
-
Suppose the city is undergoing severe ination. Specifically, both goods prices have risen by 10%. What percentage of a raise in the wage rate should Alex request from her boss, for her to maintain...
-
1. An iron cube of mass 0.55 kg is raised to a temperature of 100C by being placed in boiling water for 5 minutes. It is then removed and transferred immediately to an aluminium calorimeter filled...
-
Let A be a nonsingular 2 Ã 2 matrix with singular value decomposition A = P QT and singular values Ï1 ¥ Ï2 > 0. (a) Prove that the image of the unit (Euclidean) circle under the...
-
Suppose the spot and six-month forward rates on the Norwegian krone are Kr 5.78 and Kr 5.86, respectively. The annual risk-free rate in the United States is 3.8 percent, and the annual risk-free rate...
-
Show how you might synthesize each of the following starting with a-tetralone (Section 15.9): (a) (b) (c) (d) HO So OH 02 H5
-
Give structures (including stereochemistry where appropriate) for compounds A-G: (a) (b) (c) (d) 2 NaNH2 Benzene+ o c heat (1) Li, EtNH2 E (C9H10 (2) NHCI (Section 7.15B)) Br2 D F+ enantiomer (major...
-
Show how you might synthesize each of the following compounds starting with either benzyl bromide or allyl bromide: (a) (b) (c) (d) (e) (f) CH5 CN
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


