Which of the following compounds should have the larger energy barrier to internal rotation about the indicated
Question:
Which of the following compounds should have the larger energy barrier to internal rotation about the indicated bond?
Explain your reasoning carefully.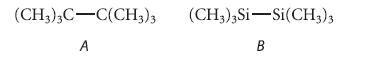
Transcribed Image Text:
(CH3)3C-C(CH3)3 (CH3)3 Si-Si(CH3)3 A B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
A major contributor to the barrier to internal rotation is the van de...View the full answer

Answered By

Jeff Omollo
As an educator I have had the opportunity to work with students of all ages and backgrounds. Throughout my career, I have developed a teaching style that encourages student engagement and promotes active learning. My education and tutoring skills has enabled me to empower students to become lifelong learners.
5.00+
5+ Reviews
52+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
From what you learned in Sec. 1.3B about the relative lengths of CC and CO bonds, predict which of the following compounds should have the larger energy difference between gauche and anti...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
Sketch a diagram of potential energy versus angle of rotation about the carboncarbon bond of chloroethane, H 3 CCH 2 Cl. The magnitude of the energy barrier to internal rotation is 15.5 kJ mol 1 (3.7...
-
Consider the many moments of joy in the movie. Why include them? What risky acts of courage do Katherine, Mary and Dorothy take? How are they rewarded? The film shows the strong relationships that...
-
The court seems to regard Joness allegations as trivial. In fact, hasnt she alleged disgusting behavior by her employer? How can the court regard her claims so lightly?
-
As described in Exercise 14.43, the decomposition of sulfuryl chloride (SO2Cl2) is a first-order process. The rate constant for the decomposition at 660 K is 4.5 10-2s-1. (a) If we begin with an...
-
2. What is a forward-looking multiple? Why should one use forward-looking multiples as opposed to backward-looking multiples when valuing companies?
-
Huggins Inc. experienced the following transactions for 2010, its first year of operations. 1. Issued common stock for $60,000 cash. 2. Purchased $210,000 of merchandise on account. 3. Sold...
-
Cumberland County Senior Services is a non-profit organization devoted to providing essential services to seniors who live in their own homes within the Cumberland County area. Three services are...
-
(a) Draw Newman projections of the most stable conformations about each of the carboncarbon bonds in the principal chain of 2,2-dimethylpentane. Use models! (b) Combine these to predict the most...
-
The anti conformation of 1,2-dichloroethane, ClCH 2 CH 2 Cl, is 4.81 kJ mol1 (1.15 kcal mol 1 ) more stable than the gauche conformation. The two energy barriers (measured relative to the energy of...
-
Find the general solution of the following differential equations. y' (t) = 2ty
-
Conservation efforts include reintroduction of species into the wild from captive breeding programs. Leung et al. (2018) rewilded mice from the inbred laboratory strain of mouse, C57BL/6, that had...
-
The ending balance of the Accounts Receivable account was \(\$ 7,800\). Services billed to customers for the period were \(\$ 21,500\), and collections on account from customers were \(\$ 23,600\)....
-
Cash Flow Activity Classification Classify each activity as financing, investing, or operating: 1. Repay a loan from a bank. 2. Sell merchandise from a storefront operation. 3. Dispose of an old...
-
Generally Accepted Accounting Principles Select the best answer to each of the following MBC) questions: 1. Accounting rules are developed to provide: a. Simplicity b. Useful information c....
-
Basic Accounting Principles Identify whether the following statements are true or false. 1. Together the revenue recognition principle and the expense recognition (matching) principle define the...
-
Distinguish between a parietal and a visceral membrane.
-
Problem 3.5 (4 points). We will prove, in steps, that rank (L) = rank(LT) for any LE Rnxm (a) Prove that rank (L) = rank (LTL). (Hint: use Problem 3.4.) (b) Use part (a) to deduce that that rank(L) =...
-
When glucose (Problem) is treated with NaBH4, reaction occurs to yield sorbitol, a polyalcohol commonly used as a food additive. Show how this reductionoccurs. CH- NABHA -2 Sorbitol Glucose
-
Give IUPAC names for the followingcompounds: (a) Br (b) (c) CH CH3CH2CHCH2CH2CH3 CHH-CH2Co CHCH2C (f) O2- (d) CH CN (e) CHCCH2CHH "Co2 CH2CH2COH
-
Draw structures corresponding to the following IUPAC names: (a) 2, 3-Dimethylhexanoic acid (b) 4-Methylpentanoic acid (c) Trans-1, 2-Cyclobutanedicarboxylic acid (d) o-Hydroxybenzoic acid (e) (9Z,...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...

Study smarter with the SolutionInn App


