5-Methylcyclopentadiene undergoes homolytic bond cleavage of a C!H bond to form a radical that exhibits five resonance
Question:
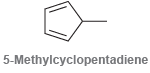
Transcribed Image Text:
5-Methylcyclopentadiene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 91% (12 reviews)
This h...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When an acyl peroxide undergoes homolytic bond cleavage, the radicals produced can liberate carbon dioxide to form alkyl radicals: Using this information, provide a mechanism of formation for each of...
-
Draw resonance structures for each of the following compounds: a. b. c. d. e. f. g. h. i. j. OH
-
Draw all significant resonance structures for each of the following compounds: a. b. c. d. e. f. g. h. i. j. k. l. . z: N.
-
When working as a tax professional, one issue that often arises is the need to provide difficult or disappointing guidance to a client. In the following example, determine the best course of action....
-
The daily demand of a product can be specified by a normal distribution. Its average daily demand is 250 units with a standard deviation of 40 units. The delivery lead time of this product is also...
-
Let f (x, y) = 3x 2 + xy + 5y 2 . Find af Jx and af dy
-
1. Explain why the value of a business may differ under different owners.
-
Mukul is a teacher at Rockland School and he runs a tennis shop called Racket's Rackets. He and his wife Nikki have combined bank interest of $1,011. Nikki made $850 in tips last year. If they get a...
-
Range Company has the following selected data from its annusl period ended December 31. Note: Use a negative sign for subtractiohs in the statement of cash flows. Frepare a statement of cash flows...
-
The average length of short hospital stays for men is slightly longer than that for women, 5.2 days versus 4.5 days. A random sample of recent hospital stays for both men and women revealed the...
-
At 298 K and 1 bar pressure, the density of water is 0.9970 g cm 3 , and C P,m = 75.3 J K -1 mol -1 . The change in volume with temperature is given by V = V i T, where , the coefficient of thermal...
-
Calculate S surroundings and S total for the processes described in parts (a) and (b) of Problem P5.16. Which of the processes is a spontaneous process? The state of the surroundings for each part is...
-
Draw a graph to show the change in the land market from 1969 to 2007. Explain how the moratorium will influence the land market in 2008. Use the following information to work Problems 8 and 9....
-
As shown on the attached chart, what is the approximate current 7-year spread premium for Kellogg Bonds? 25 Basis Points 75 Basis Points 200 Basis Points AUS Treasury Actives Curve X-ads Tenor...
-
A pharmaceutical company claims to have invented a new pill to aid weight loss. They claim that people taking these pills will lose more weight than people not taking them. A total of twenty people...
-
Let U = {a, b, c, d, e, f} be the universal set and let A = {a, b, c, d, e, f}. Write the set A. Remember to use correct set notation. Provide your answer below: A=
-
Produce a poster series of three (3) A3 sized posters on creativity in the early years. As a collective the poster series must articulate the importance of aesthetics and creativity for young...
-
Find the second derivative of the function. g(x) = ex In(x) g"(x) = Need Help? Read It
-
If C(x) = 15x + 2000 calculates the cost in dollars of producing x radios, interpret the numbers 15 and 2000 in the formula for C(x).
-
Find the equation of the plane passing through the points P 5,4,3 ,Q 4,3,1 and R 1,5,4
-
One of the reactions used in determining the sequence of nucleotides in a strand of DNA is reaction with hydrazine. Propose a mechanism for the following reaction, which occurs by an initial...
-
Which compound in each of the following pairs is more basic? (a) CH 3 CH 2 NH 2 or CH 3 CH 2 CONH 2 (b) NaOH or CH 3 NH 2 (c) CH 3 NHCH 3 or pyridine
-
What product would you expect from Hofmann elimination of a heterocyclic amine such as piperidine? Write all thesteps? Piperidine
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


