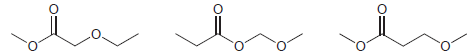
A 1 H NMR spectrum was acquired for each of the following constitutional isomers. Comparison of the
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
HH methylene proto...View the full answer

Answered By

Stephanie Olivero
No, Education & Tutoring Experience
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify one possible research problem for each of the following decision problems. Tell whether the decision problems are discovery-oriented or strategy-oriented. a. Why have sales of my brand...
-
Identify the best answer for each of the following 1. Which of the following statements concerning the accounting and financial reporting for capital assets is false? a. Capitalization thresholds...
-
Identify the location strategies for each of the following types of retailers. Department sorest Specialty apparel stores Category killers/specialists Grocery stores
-
Mario and Kaitlin are married and file a joint tax return. They have adjusted gross income of $385,000 that includes $4,700 of investment income ($3,000 short-term capital gains and $1,700 of...
-
Using the basic expectations theory, describe how the shape of the yield curve is determined.
-
Zowie was interested in starting a magazine catering to alternative lifestyles. Wanting to avoid the double taxation inherent in the corporate form of carrying on a business but still having the...
-
What is the need for preparing a bank reconciliation statement?
-
Consider the business event processing activity, entering a customers order. Identify the key business event data ( who, what, where, and when) you would want to capture.
-
Required information The Foundational 1 5 ( Algo ) [ LO 1 2 - 1 , LO 1 2 - 2 , L 0 1 2 - 3 , LO 1 2 - 5 , LO 1 2 - 6 ] [ The following information applies to the questions displayed below. ] Cardinal...
-
A digital audio system is designed to minimize the effect of disturbances as shown in Figure E4.2. As an approximation, we may represent G(s) = K2. (a) Calculate the sensitivity of the system due to...
-
Predict the chemical shifts for the signals in the 1 H NMR spectrum of each of the following compounds: (a) (b) (c) (d) (e)
-
On January 2, 2007, a Sunny Communications $1,000 face value, six-year bond sold for $889. Investors who bought this particular bond will be paid interest equal to $40 every six months. Market...
-
The pH in 0.10 M CH 3 CH 2 COOH(aq) must be (a) Equal to [H 3 O + ] in 0.10 M HNO 2 (aq); (b) Less than the pH in 0.10 M HI(aq); (c) Greater than the pH in 0.10 M HBr(aq); (d) Equal to 1.0.
-
Write a brief statement that interprets the confidence interval. Choose the correct answer below. A. There is a 99% chance that the true value of the population mean weight of newborn girls will fall...
-
Transcribed image text: If estimated annual factory overhead is $1,072,500; overhead is applied using direct labor hours, estimated annual direct labor hours are 275,000 actum March factory overhead...
-
Your firm has limited capital to invest and is therefore interested in comparing projects based on the profitability index (PI), as well as other measures. What is the PI of the project with the...
-
The following rates are applicable to annual payroll in British Columbia Question 17 options: 1234 1.95% x total B.C. remuneration 1234 2.925% x (B.C. remuneration - $500,000) 1234 Tax Rate 1234...
-
Assume that different groups of couples use a particular method of gender selection and each couple gives birth to one baby. This method is designed to increase the likelihood that each baby will be...
-
Approximate to the nearest thousandth. log, 18
-
Consider the discrete group G of order 8 that has the following Cayley diagram e If we have the sequence of operations: fcagec, which of the options represents the reduction of the sequence to a...
-
Consider 2-methylbutane (isopentane) Sighting along the C2C3 bond: (a) Draw a Newman projection of the most stable conformation. (b) Draw a Newman projection of the least stable conformation. (c)...
-
What are the relative energies of the three possible staggered conformations around the C2C3 bond in 2, 3-dimethylbutane? (See Problem 3.42)
-
Construct a qualitative potential-energy diagram for rotation about the CC bond of 1, 2-dibromoethane. Which conformation would you expect to be more stable? Label the anti and gauche conformations...
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


