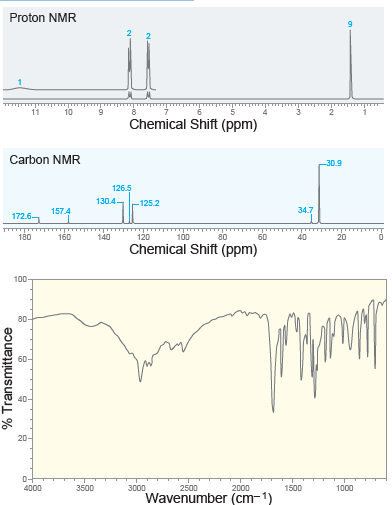
A compound with molecular formula C 11 H 14 O 2 exhibits the following spectra ( 1
Question:
Transcribed Image Text:
Proton NMR 11 Chemical Shift (ppm) Carbon NMR 30.9 126.5 130.4- -125.2 34.7 157.4 172.6 120 100 80 180 160 140 60 40 Chemical Shift (ppm) 100 80- По 60- 40- 20- 0- 4000 3500 3000 2500 2000 1500 1000 Wavenumber (cm-1) % Transmittance
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)

Answered By

Gauri Hendre
I worked as EI educator for Eduphy India YT channel. I gave online tutorials to the students who were living in the villages and wanted to study much more and were preparing for NEET, TET. I gave tutions for topics in Biotechnology. I am currently working as a tutor on course hero for the biochemistry, microbiology, biology, cell biology, genetics subjects. I worked as a project intern in BAIF where did analysis on diseases mainly genetic disorders in the bovine. I worked as a trainee in serum institute of India and Vasantdada sugar institute. I am working as a writer on Quora partner program from 2019. I writing on the topics on social health issues including current COVID-19 pandemic, different concepts in science discipline. I learned foreign languages such as german and french upto A1 level. I attended different conferences in the science discipline and did trainings in cognitive skills and personality development skills from Lila Poonawalla foundation. I have been the member of Lila poonawalla foundation since 2017. Even I acquired the skills like Excel spreadsheet, MS Office, MS Powerpoint and Data entry.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When a compound with molecular formula C11H14O2 undergoes acid-catalyzed hydrolysis, one of the products that is isolated gives the following 1H NMR spectrum. Identify the compound. 10 9 4
-
A compound with molecular formula C4H6O gives the infrared spectrum shown in Figure 13.34. Identify the compound. 3.5 4 5 12 13
-
Identify the following compounds. (Relative integrals are given from left to right across the spectrum.) a. The 1H NMR spectrum of a compound with molecular formula C4H10O2 has two singlets with an...
-
The passage indicates that the late 1850s Democrats: F. Were all Southern slaveholders who wanted to expand slavery into the territories. G. Used legislation in the early 1850s to support their...
-
What is breach of contract? What remedies are available to a party injured by a breach?
-
Find the value of the line integral If F is conservative, the integration may be easier on an alternative path.) (a) r(t) = ti + tj, 0 t 2 (b) r(t) = 2 cos ti + 2 sin tj, 0 t /2 C F. dr.
-
Which of the seven elements of the service marketing mix are most important in the LA Galaxy marketing program?
-
a. Identify the physical control weaknesses depicted in the flowchart for Problem 6. b. Describe the IT controls that should be in place in thissystem. Customer Sales Department Billing Accounts...
-
Stellar Inc. issued 20,000 shares of common stock, $5 par value, for $28 per share on March 28, 2020. Related to this transaction, Stellar incurred legal and administrative costs totaling $5,000....
-
A Student object should validate its own data. The client runs this method, called validateData(), with a Student object, as follows: String result = student.validateData(); if (result == null) else...
-
An ideal gas in a piston and cylinder assembly with adiabatic walls undergoes an expansion against a constant external pressure. Are S, S surroundings , and S total positive, negative, or zero?...
-
Is the equation valid for an ideal gas? Tf PV; -V;) T; Cy dT Lav = C, n2v, -v) %3D AS =
-
The caloric consumption of 32 American adults was measured and found to average 2,157. Assume the population standard deviation is 260 calories per day. Construct confidence intervals to estimate the...
-
Go to: https://www.instagram.com/ryderseyewear/ on your desktop, laptop, or mobile (or a combination of all 3). You are the new Social Media Marketing Manager for Ryders Eyewear. You've been asked...
-
As leaders, it is very important that we have the ability to assess our own motivation and the motivation of others around us. It is also important to recognize the key factors involved in...
-
At the end of this exam, you will find Article 1 - " How Companies Can Prepare for a Long Run of High Inflation ". Please read the article and, when necessary, consult additional sources and the...
-
You can develop your capabilities as a manger by better understanding different ways of motivating and rewarding employees. You can also better prepare for your own career by better understanding the...
-
Topic: Project Malasakit of Kara David https://projectmalasakit.org/ What is the pros and cons of these alternative courses of the action below: Strengthen the internal organization via promoting it...
-
Solve each polynomial inequality in Exercises 142 and graph the solution set on a real number line. Express each solution set in interval notation. x3 > 9x2
-
Describe a group you belong or have belonged discuss the stages of group development and suggest how to improve the group effectiveness by using the group development model.
-
What is the expected substitution product (including its stereochemical configuration) in the SN2 reaction of potassium iodide in acetone solvent with the following compound? (D = 2H = deuterium, an...
-
(a) Give the structure of the S*2 reaction product between ethyl iodide and potassium acetate. H,C-C potassium acetate
-
(a) Give the structure of the S*2 reaction product between ethyl iodide and potassium acetate. H,C-C potassium acetate
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.
-
On August 1 , 2 0 2 3 , Mark Diamond began a tour company in the Northwest Territories called Millennium Arctic Tours. The following occurred during the first month of operations: Aug. 1 Purchased...

Study smarter with the SolutionInn App


