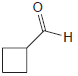
Assign a systematic (IUPAC) name to each of the following compounds: (a) (b) (c) (d) (e) Br
Question:
(a)
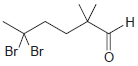
(b)
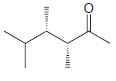
(c)
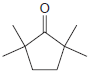
(d)
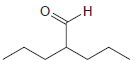
(e)
Transcribed Image Text:
Н Br Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
a 55dibromo22dimethylhexan...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Name the following compounds: (a) (b) (c) (d) (e) (f) (g) (h) CH,CH,C CH OH CH CH CH NO COOH Br OCH(CH)2 OH NO NO CH,OCH,CH,
-
Name the following compounds: (a) (b) (c) (d) (e) (f) (g) (h) CI Cl NO, OCH COOH CH,( OCH COOH Cl OH CI CI Cl CH CHCH,CH, CHO BFi
-
Give the IUPAC name of each of the following compounds. a. b. c. d. CH3CHCH2COOH CH3 CH CHCH CH3 O CH CH CHCCH CH CH2 CH CH3
-
Ebbers Corporation overstated its ending inventory balance by $15,000 in the current year. What impact will this error have on cost of goods sold and gross profit in the current year and following...
-
Which of the following is not an example of a decision or informed judgment that a potential investor would make from accounting information? Select one: a. Future profitability based on past...
-
Perform the indicated operations. Leave the result in polar form. 245.6/326.44 17.19/192.83
-
Why was the third-party registration system established for ISO 9000 certification? LO.1
-
A solar collector consists of a parallel plate channel that is connected to a water storage plenum at the bottom and to a heat sink at the top. The channel is inclined 0 = 30 from the vertical and...
-
Looking for a solution to the following question Match each description 1 through 4 with the characteristic of preferred stock that it best describes. Characteristic Description 1. Receives current...
-
Imagine you are a member of the team at the investors reviewing the viability of the Zopa business. On which criteria would you assess the future potential of the business and the returns in your...
-
When N,N-dimethylaniline is treated with bromine, ortho and para products are observed. Yet, when N,Ndimethylaniline is treated with a mixture of nitric and sulfuric acid, only the meta product is...
-
Draw the structure of each of the following compounds: (a) (S)-3,3-dibromo-4-ethylcyclohexanone (b) 2,4-dimethyl-3-pentanone (c) (R)-3-bromobutanal
-
How do economists use the multiplier?
-
As an official sponsor of the Olympics, what specific benefit did John Hancock use to help drive sales in their national offices?
-
assumes that Nia has both a discount rate of zero and faces an interest rate of zero. These assumptions made calculating her constant level of consumption expenditure of $56,000 fairly...
-
Paul Petersen lives in Northern California. He owns a BMW car worth about $20,000. He wants to take a trip to Nevada with his girlfriend Patricia, who lives in Los Angeles. He takes his car into...
-
Do you see gendered patterns of interaction in personal relationships? Does knowing about gender linked patterns affect how other interpret on what happens in a relationships?
-
Significance For bone density scores that are normally distributed with a mean of 0 and a standard deviation of 1, find the percentage of scores that are significantly high (or at least 2 standard...
-
If one movie ticket costs $13.50, how much will y tickets cost?
-
Draw and label the E and Z isomers for each of the following compounds: 1. CH3CH2CH==CHCH3 2. 3. 4. CH,CH2C CHCH2CH Cl CH3CH2CH2CH2 CH CH2CCCH2CI CHCH3 CH3 HOCH CH CCC CH O-CH C(CH
-
How many valence electrons does each of the following atoms have? (a) Na (b) Cl (c) Si (d) B (e) Ne (f) N
-
Add any missing unshared electron pairs (if any), then, using curved arrows to show the shifts in electrons, write the contributing resonance structures and resonance hybrid for each of the...
-
For each set of resonance structures that follow, add a curved arrow that shows how electrons in the left formula shift to become the right formula, and designate the formula that would contribute...
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


