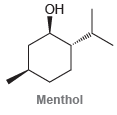
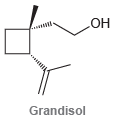
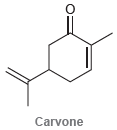
Circle the isoprene units in each of the following compounds. (a) (b) (c) Menthol HO Grandisol
Question:
(a)
(b)
(c)
Transcribed Image Text:
ОН Menthol HO Grandisol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
a b...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Locate the isoprene units in each of the monoterpenes, sesquiterpenes, and diterpenes shown in Figure 26.6. (In some cases there are two equally correct arrangements.)
-
Identify the isoprene units in each of the following naturally occurring substances: (a) Ascaridole, a naturally occurring peroxide present in chenopodium oil:
-
(a) Show the isoprene units in each of the following terpenes. (b) Classify each as a monoterpene, sesquiterpene, diterpene, and so on. CH Zingiberene (from oil of ginger (from oil of celery)...
-
a) Assuming that your capital is constrained, what is the fifth project that you should invest in? Please show work. b) Assuming that your capital is constrained so that you only have $600,000...
-
Selected production and cost data of Laura's Caliper Co. follow for May 2018: On May 31, the Mixing Department ending Work-in-Process Inventory was 80% complete for materials and 45% complete for...
-
In petroleum refining, hydrocarbons are often manipulated by reacting them with H 2 (g). If hexene, C 6 H 12 , is reacted with hydrogen to form hexane, C 6 H 14 , how many moles of hydrogen are...
-
Vasanth Ltd manufactures product A, which yields two by-products B and C. The actual joint expense of manufacture for a period was Rs 8,000. Subsequent expenses and other data are as follows: A (Rs)...
-
1. Why do you think so many American citizens and lawmakers reacted negatively to Googles decision in 2006? 2. Does the fact that Google is an Internet company change societal expectations of it...
-
Exercise 9-15 Oriole Legler requires an estimate of the cost of goods lost by fire on March 9. Merchandise on hand on January 1 was $33,440. Purchases since January 1 were $63,360; freight-in,...
-
Alaska Airlines is unique among the nine major U.S. carriers not only for its extensive flight coverage of remote towns throughout Alaska (it also covers the U.S., Hawaii, and Mexico from its primary...
-
Classify each prostaglandin according to the instructions provided in Section 26.7. In section 26.7 ¢ Prostaglandins are biochemical regulators that are even more powerful than steroids. ¢...
-
Determine whether each of the following compounds is a terpene. (a) (b) (c) (d)
-
Solve Prob. 8?132 if the force P is applied horizontally to the right. 120 mm P.
-
Alvarado Company produces a product that requires 5 standard direct labor hours per unit at a standard hourly rate of $12.00 per hour. If 5,700 units used 29,400 hours at an hourly rate of $11.40 per...
-
7. (30 points) You are a teaching assistant (TA) for a new course in the department and you wish to measure the amount of time that students spend engaging with the online resources. Using the Canvas...
-
Mod Clothiers makes women's clothes. It costs $28,000 to produce 5,000 pairs of polka-dot polyester pants. They have been unable to sell the pants at their usual price of $50.00. The company is...
-
In a mid-sized manufacturing company, the annual financial statements were prepared for audit by an external auditing firm. The company\'s finance team had diligently compiled the financial data, and...
-
Explain the meaning of the SMART acronym. In 100-200 words, define what the words "goal" and "success" mean to you. Summarize your thoughts on whether or not the SMART model can help you become a...
-
Evaluate the following integrals as they are written. px 1.5. 0 0 2e-** dy dx
-
A local politician is concerned that a program for the homeless in her city is discriminating against blacks and other minorities. The following data were taken from a random sample of black and...
-
An unknown compound gives the following mass, IR, and NMR spectra. Propose a structure, and show how it is consistent with the spectra. Show the fragmentations that give the prominent peaks at m/z...
-
Hexahelicene seems a poor candidate for optical activity because all its carbon atoms are sp2 hybrids and presumably flat. Nevertheless, hexahelicene has been synthesized and separated into...
-
Draw just the bonding -MO's for the cycloheptatrienyl cation. Draw the energy diagram to show the relative energies of all the MO's, and show which orbitals the electrons would occupy in the ground...
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


