Compound Y has molecular formula C 7 H 12 . Hydrogenation of compound Y produces methylcyclohexane. Treatment
Question:
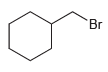
Predict the products when compound Y undergoes ozonolysis.
Transcribed Image Text:
Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
Br HBr ROO...View the full answer

Answered By

Atuga Nichasius
I am a Highly skilled Online Tutor has a Bachelor’s Degree in Engineering as well as seven years of experience tutoring students in high school, bachelors and post graduate levels. I have a solid understanding of all learning styles as well as using asynchronous online platforms for tutoring needs. I individualise tutoring for students according to content tutoring needs assessments.
My strengths include good understanding of all teaching methods and learning styles and I am able to convey material to students in an easy to understand manner. I can also assists students with homework questions and test preparation strategies and I am able to help students in math, gre, business , and statistics
I consider myself to have excellent interpersonal and assessment skills with strong teaching presentation verbal and written communication
I love tutoring. I love doing it. I find it intrinsically satisfying to see the light come on in a student's eyes.
My first math lesson that I taught was when I was 5. My neighbor, still in diapers, kept skipping 4 when counting from 1 to 10. I worked with him until he could get all 10 numbers in a row, and match them up with his fingers.
My students drastically improve under my tutelage, generally seeing a two grade level improvement (F to C, C to A, for example), and all of them get a much clearer understanding!
I am committed to helping my students get the top grades no matter the cost. I will take extra hours with you, repeat myself a thousand times if I have to and guide you to the best of my ability until you understand the concept that I'm teaching you.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Compound A has molecular formula C 7 H 12 . Hydrogenation of compound A produces 2 methylhexane. Hydroboration-oxidation of compound A produces an aldehyde. Draw the structure of compound A, and draw...
-
Compound X has molecular formula C 7 H 14 . Hydrogenation of compound X produces 2, 4-dimethylpentane. Hydroboration-oxidation of compound X produces a racemic mixture of 2, 4-dimethylpentan-1-ol...
-
An alkene X with molecular formula C7H12 adds HBr to give a single alkyl halide I with molecular formula C7H13Br and undergoes catalytic hydrogenation to give 1,1-dimethylcyclopentane. Draw the...
-
As the traffic manager for ABC Electronics, you have been charged with the task of reducing shipping costs for a fast selling cable product that is sold by the pound. You have a very satisfactory...
-
"Packaging must be terribly important today on lots of products. We spend a fortune on it. I read recently about a detergent packaging gimmick--an 'overcap.' It goes onto a bottle, over the regular...
-
In Exercises evaluate the definite integral using the formulas from Theorem 5.20. Data from in Theorem 5.20 THEOREM 5.20 Differentiation and Integration Involving Inverse Hyperbolic Functions Let u...
-
8C3
-
Coulombs Law states that the force of attraction between two charged particles is directly proportional to the product of the charges and inversely proportional to the square of the distance between...
-
A stock is expected to pay a dividend of $3.00 at the end of the year (i.e., D1 = $3.00), and it should continue to grow at a constant rate of 7% a year. If its required return is 12%, what is the...
-
The file contains the average price of a room at two-star, three-star, and four-star hotels in cities around the world in 2009 in English pounds (about US$1.57 as of October 2010). Complete the...
-
Consider an oligopoly industry whose firms have identical demand and cost conditions. If the firms decide to collude, then each one will want to produce the amount of output that it would if it were:...
-
Predict the products for each of the following transformations. a. b. aso heat 1) TsCl, py -OH 2) NaOEt
-
Why is goal congruence important? lp6
-
As part of the study on ongoing fright symptoms due to exposure to horror movies at a young age, the following table was presented to describe the lasting impact these movies have had during bedtime...
-
Exercise 1.10: State space realization Define a state vector and realize the following models as state space models by hand. One should do a few by hand to understand what the Octave or MATLAB calls...
-
Solve: (5)*+1 = 25x
-
The ball bearing made of steel is to be heat treated. It is heated to a temperature of 620C and then quenched in water that is at a temperature of 50C. The ball bearing has a diameter of 5 cm and the...
-
1. Using the net present value? method, calculate the comparative cost of each of the three payment plans being considered by New Med 2. Which payment plan should New Med choose? Explain. 3. Discuss...
-
Find the domain of f and write it in set- builder or interval notation. f(x) = log(4- V2-x)
-
In your readings, there were many examples given for nurturing close family relationships in this ever-evolving technological society we live in Based upon your readings and research describe three...
-
A Follow the flow of electrons indicated by the curved arrows in each of the following reactions, and predicts the products thatresult: (b) :0- (a) -: "CH
-
When isopropylidenecyclohexane is treated with strong acid at room temperature, isomerization occurs by the mechanism shown below to yield 1-iso- propylcyclohexenc: At equilibrium, the product...
-
Add curved arrows to the mechanism shown in Problem 5.32 to indicate the electron movement in each step.
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


