Consider the following reaction: a. Draw the mechanism of this reaction. b. What is the rate equation
Question:
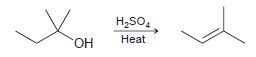
a. Draw the mechanism of this reaction.
b. What is the rate equation of this reaction?
c. Draw an energy diagram of the reaction.
Transcribed Image Text:
H2SO, Heat OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
a b This is an E1 pro...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following reaction at 800. K: N2(g) + 3F2(g) 2NF3(g) An equilibrium mixture contains the following partial pressures: PN2 = 0.021 atm, PF2 = 0.063 atm, and PNF3 = 0.48 atm. Calculate Go...
-
Consider the following reaction at equilibrium: From the data shown here, calculate the equilibrium constant (both KP and Kc) at each temperature. Is the reaction endothermic or exothermic?...
-
Consider the following reaction at 25C: Fe(OH)2 Fe2+(aq) + 2OH-(aq) Calculate G for the reaction. Ksp for Fe(OH)2 is 1.6 10-14.
-
rewrite/downside Integrity and credibility are the ethics of professional practice that Juan Gomez was lacking in this instance. Juan Gomez lacked integrity because he created a conflict of interest...
-
Ethical Considerations" Please respond to the following: Reflect upon the responsibilities placed on auditors by the PCAOB, and discuss whether those expectations are adequate considering current...
-
Factor the given expressions completely. x 10 x 2
-
What are some of the different ways Mountain Dew can assess the success of its campaign?
-
A publishing house publishes three weekly magazinesDaily Life, Agriculture Today, and Surfs Up. Publication of one issue of each of the magazines requires the following amounts of production time and...
-
10. The following data are taken from the cash-basis accounting records of Myerson Company for the year ended December 31, 2021: Selected Data as of December 31, 2021 Customers billed in 2021 for...
-
For the spring assemblages shown in Figures P2-8 through P2-16, determine the nodal displacements, the forces in each element, and the reactions. Use the direct stiffness method for all problems....
-
A purely competitive firm whose goal is to maximize profit will choose to produce the amount of output at which: a. TR and TC are equal. b. TR exceeds TC by as much as possible. c. TC exceeds TR by...
-
If it is possible for a perfectly competitive firm to do better financially by producing rather than shutting down, then it should produce the amount of output at which: a. MR < MC. b. MR = MC. c. MR...
-
Predict the product of the following reaction:
-
Share your thoughts on the descriptions of coaching versus mentoring. Discuss which technique you personally find more helpful, incorporating your peers' example scenarios if possible. Provide...
-
Hanung Corp has two service departments, Maintenance and Personnel. Maintenance Department costs of $380,000 are allocated on the basis of budgeted maintenance-hours. Personnel Department costs of...
-
Discuss difference between nominal interest rate and real interest rate. Explain why real interest rate is more important than the nominal interest rate using your answer to Question 1 of the...
-
Refer to Figure 14-1. How would an increase in the money supply move the economy in the short and long run?
-
1) Special Relativity. Statement: Imagine this situation: Alice stands in New York City while Bob, aboard a plane departing from Boston, directly crosses over Alice at t=0. Disregard the vertical...
-
In Exercises 17 through 22, you are given the price p(q) at which q units of a particular commodity can be sold and the total cost C(q) of producing the q units. In each case: (a) Find the revenue...
-
In Problem use geometric formulas to find the unsigned area between the graph of y = f(x) and the x axis over the indicated interval. f(x) = x + 5; [0, 4]
-
In the DebyeHckel theory, the counter charge in a spherical shell of radius r and thickness dr around the central ion of charge +Q is given by Q 2 re r dr. Calculate the radius at which the counter...
-
Calculate the solubility of CaCO 3 (K sp = 3.4 10 -9 ) a. In pure H 2 O. b. In an aqueous solution with I = 0.0250 mol kg 1 . For part (a), do an iterative calculation of and the solubility until...
-
Calculate the probability of finding an ion at a distance greater than 1/ from the central ion.
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


