Consider the following reaction: (a) Use Table 6.1 to estimate ÎH for this reaction. (b) ÎS of
Question:
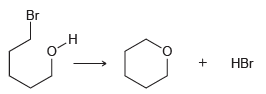
(a) Use Table 6.1 to estimate ΔH for this reaction.
(b) ΔS of this reaction is positive. Explain.
(c) Determine the sign of ΔG.
(d) Is the sign of ΔG dependent on temperature?
(e) Is the magnitude of ΔG dependent on temperature?
Transcribed Image Text:
Br o-н HBr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
a Bonds Broken kJmol Bonds Formed kJmol RCH 2 x Br ...View the full answer

Answered By

Rajat Gupta
used to take tution classes from my school time.
Conducted special topic claases during my graduation to help the students pass their exams.
Currently, teaching and conducting online claases during my post- graduation too.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following reaction at some temperature: H2O(g) + CO(g) H2(g) + CO2(g) K = 2.0 Some molecules of H2O and CO are placed in a 1.0- L container as shown below. When equilibrium is reached,...
-
Consider the following reaction between oxides of nitrogen: NO2(g) + N2O(g) 3 NO (g) (a) Use data in Appendix C to predict how G for the reaction varies with increasing temperature. (b) Calculate G...
-
Consider the following reaction at 298 K: 2H2(g) + O2(g) 2H2O(l) H = 2571.6 kJ/mol Calculate Ssys, Ssurr, and Suniv for the reaction.
-
Evaluate the limit of the sequence or state that it does not exist. an || u8 n!
-
Suppose that you are sole proprietor presenting to a group of investors where you are seeking 20 million dollars to raise capital for your manufacturing company. Choose the one form of organization...
-
(a) Find (x + 5) 2 dx in two ways: (i) By multiplying out (ii) By substituting w = x + 5 (b) Are the results the same? Explain.
-
List out the advantages of calculating MHR
-
A process consists of two stirred tanks with Input q and outputs T1 and T2 (see figure). To test the hypothesis that the dynamics in each tank are basically first order, a step change in q is made...
-
On 1/1/19 Tommy Inc., a calendar year company, is created with an equity investment of $191. On the same day the company purchases 1 share of stock in another company for $106. The 1 share of stock...
-
A communication system sends data in the form of packets of fixed length. Noise in the communication channel may cause a packet to be received incorrectly. If this happens, then the packet is...
-
In each of the following cases compare the bonds identified with red arrows, and determine which bond you would expect to have the largest bond dissociation energy: a. b. CI .F Br
-
Consider the following four energy diagrams: (a) Which diagrams correspond with a two-step mechanism? (b) Which diagrams correspond with a one-step mechanism? (c) Compare energy diagrams A and C....
-
Preparation of process accounts with all output fully completed A product is manufactured by passing through three processes: A, B and C. In process C a byproduct is also produced which is then...
-
Mijka Company was started on January 1, Year 1. During Year 1, the company experienced the following three accounting events: 1. earned cash revenues of $32,500 2. paid cash expenses of $14,500 3....
-
Q2. Find the equations of the tangent and normal to the curve x3 + y = 2 at (1, 1). Q3. Find if y dx y= :xsinx + (sinx)cosx [10] [10]
-
Assume you have been given $400,000 CAD with access to all listed stocks, bonds, futures, and options worldwide. You can trade in options and futures, in combination with the underlying asset....
-
The formula weight (FW) of a gas can be determined using the following form of the ideal gas law FW = g R T / PV where g is the mass in grams, R is the gas constant, T is the temperature in Kelvin, P...
-
Consider a game in which a fair die is thrown. The player pays $5 to play and wins $2 for each dot that appears on the roll. Define X = number on which the die lands, and Y = player's net profit...
-
In Exercises 7792, use the graph to determine a. The functions domain; b. The functions range; c. The x-intercepts, if any;d. The y-intercept, if any; e. The missing function values, indicated by...
-
If 2 5 9 - k 5 8 = 2 5 8 , what is the value of k?
-
What product would you expect to obtain from the base-catalyzed Michael reaction of (a) 1, 3-diphenylprop-2-en-1-one (Section 19.5A) and acetophenone (b) 1, 3-diphenylprop-2-en-1-one and...
-
Since the products obtained from Claisen condensations are b-keto esters, subsequent hydrolysis and decarboxylation of these products give a general method for the synthesis of ketones. Show how you...
-
When acrolein (propenal) reacts with hydrazine, the product is a dihydropyrazole: Suggest a mechanism that explains this reaction. H + H2N-NH2 Acrolein Hydrazine A dihydropyrazole
-
If John invested $20,000 in a stock paying annual qualifying dividends equal to 4% of his investment, what would the value of his investment be 5 years from now? Assume Johns marginal ordinary tax...
-
help asap please!
-
Please, help asap! I have one day. Feedback will be given. & show some work. [in Excel] For the final project you will need you to create a spreadsheet /proforma of the cash flows from a property....

Study smarter with the SolutionInn App


