Consider the relative energy diagrams for four different processes: (a) Compare energy diagrams A and D. Assuming
Question:
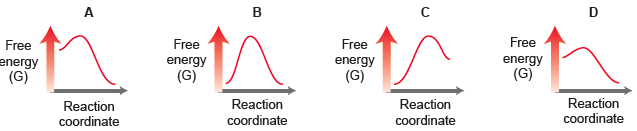
(a) Compare energy diagrams A and D. Assuming all other factors (such as concentrations and temperature) are identical for the two processes, identify which process will occur more rapidly. Explain.
(b) Compare energy diagrams A and B. Which process will more greatly favor products at equilibrium? Explain.
(c) Do any of the processes exhibit an intermediate? Do any of the processes exhibit a transition state? Explain.
(d) Compare energy diagrams A and C. In which case will the transition state resemble reactants more than products? Explain.
(e) Compare energy diagrams A and B. Assuming all other factors (such as concentrations and temperature) are identical for the two processes, identify which process will occur more rapidly. Explain.
(f) Compare energy diagrams B and D. Which process will more greatly favor products at equilibrium? Explain.
(g) Compare energy diagrams C and D. In which case will the transition state resemble products more than reactants? Explain.
Step by Step Answer:






