Consider the three constitutional isomers of dioxane (C 4 H 8 O 2 ): One of these
Question:
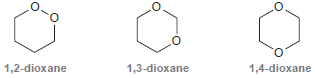
One of these constitutional isomers is stable under basic conditions as well as mildly acidic conditions and is therefore used as a common solvent. Another isomer is only stable under basic conditions but undergoes hydrolysis under mildly acidic conditions. The remaining isomer is extremely unstable and potentially explosive. Identify each isomer, and explain the properties of each compound.
Transcribed Image Text:
1,3-dioxane 1,2-dioxane 1,4-dioxane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
12dioxane has two adjacent oxygen atoms and is therefore a peroxide Like other peroxides it is ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the three constitutional isomers that have the formula C 5 H 12 . TITI H-C-C-CC CC-H 11 1 II H H=C-H H H-C-C T 1 1 -C-C-H 2 | H-- H=C=C -CC-H 3
-
Write structural formulas for at least three constitutional isomers with the molecular formula CH3NO2. (In answering this question you should assign a formal charge to any atom that bears one.)
-
Write structural formulas of the type indicated: (a) Bond-line formulas for seven constitutional isomers with the formula C4H10O; (b) Condensed structural formulas for two constitutional isomers with...
-
Factor by grouping. x 2 + 3x - 3y - xy
-
Write an essay on "Gender and Equality"
-
5. Let two planes be given by 2xy +z = 8 and z = x+y5 (a) Find the angle between the two planes. Leave your answer in degrees and round to the nearest tenth. (b) Find the vector equation of the line...
-
17. In this problem we consider whether parity is violated by any of the option prices in Table 1. Suppose that you buy at the ask and sell at the bid, and that your continuously compounded lending...
-
(Multiple-Choice) 1. Which of the following is a characteristic of a corporation? a. No income tax b. Mutual agency c. Limited liability of stockholders d. Both a and b 2. Home Team, Inc., issues...
-
Required Information [The following information applies to the questions displayed below.) Blue Skles Equipment Company uses the aging approach to estimate bad debt expense at the end of each...
-
It's amazing how much difference there is in the way proposals are presented at two different firms," said John Woods to his assistant, Pete Madsen, as he pointed to the stack of capital investment...
-
Noting marked increases in weight across the population, researchers, nutritionists, and physicians have struggled to find ways to stem the tide of obesity in many Western countries. They have...
-
For decades, researchers, politicians, and tobacco company executives debated the relation between smoking and health problems such as cancer. a. Why was this research necessarily correlational in...
-
SewFun Company produces two models of sewing basket. Information about SewFuns products is given below: SewFuns fixed costs total $35,200. Required: 1. Determine SewFuns weighted average contribution...
-
int rFibNum(int a, int b, int n) { if(n == 1) return a; else if( n == 2) return b; else return rFibNum(a,b, n-1) + rFibNum(a, b, n-2); } In the code above; a) how many base cases are there? b) what...
-
Watch the Super Nanny (i.e., Jo Frost) episode "The Orm Family" (Season 1, Episode 3) and answer the following questions. Unless otherwise specified, your answers should focus on Declan (the 3 year...
-
2. Suppose Ford officials were asked to justify their decision. What moral principles do you think they would invoke? Assess Ford's handling of the Pinto from the perspective of each of the moral...
-
2. You have been asked to design the proto-type of an Automatic Grocery Vending Machine 10 (AGVM) for the super store. Automatic Grocery Vending Machine (AGVM) is a machine where different types of...
-
1. What does Porter's 5 Forces analysis strategy do? 2. Do most people agree Why? or disagree with this aspect Why? Here is the reference video, https://www.youtube.com/watch?v=Dfp23xSqpdk 3. What...
-
Solve the rational inequality 5 4 + 2x V 0
-
2. In the circuit given in Figure 2, i,(t) = 5.67cos(5t)A and v (t) = 70.71 cos(5t 60) V a) Find the equivalent load impedance. State whether the load is inductive or capacitive. b) Calculate the...
-
Give the configuration of each asymmetric atom in the following compounds. (a) (b) meso-3,4- dimethlylhexane CH H,C H OH
-
(a) Using lines, wedges, and dashed wedges as appropriate, draw perspective structures of the two stereoisomers of ibuprofen, a well-known non steroidal anti-inflammatory drug. (b) Only the S...
-
Draw the structure of the chiral cyclic alkane of lowest molecular mass. (No isotopes are allowed.)
-
Problem 12.6A (Algo) Liquidation of a partnership LO P5 Kendra, Cogley, and Mel share income and loss in a 3.21 ratio (in ratio form: Kendra, 3/6: Cogley, 2/6; and Mel, 1/6), The partners have...
-
Melody Property Limited owns a right to use land together with a building from 2000 to 2046, and the carrying amount of the property was $5 million with a revaluation surplus of $2 million at the end...
-
Famas Llamas has a weighted average cost of capital of 9.1 percent. The companys cost of equity is 12.6 percent, and its cost of debt is 7.2 percent. The tax rate is 25 percent. What is the companys...

Study smarter with the SolutionInn App


