Draw a plausible mechanism for each of the following reactions: (a) (b) HO HO- [H2SO4] -H20
Question:
Draw a plausible mechanism for each of the following reactions:
(a)
![HO HO- [H2SO4] -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1606/7/3/1/3595fc4c65f2b52c1606731358604.jpg)
(b)
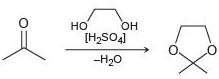
Transcribed Image Text:
HO HO- [H2SO4] -H20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)
a b HO HEOSOTH ...View the full answer

Answered By

Surojit Das
I have vast knowledge in the field of Mathematics, Business Management and Marketing. Besides, I have been teaching on the topics Management leadership, Business Administration, Human Resource Management, Business Communication, Accounting, Auditing, Organizer Behaviours, Business Writing, Essay Writing, Copy Writing, Blog Writing since 2020. It is my personality to act quickly in any emergency situations when students need my services. I am very professional and serious in every questions students asked me at the time of dealing any projects. I have been serving detailed, quality, properly analysed research paper through the years.
4.80+
91+ Reviews
279+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw a plausible mechanism for each of the following transformations: (a) (b) [TSOH] MENH2 -H20 Et [TSOH] EENH2 -H20
-
Draw a plausible mechanism for each of the following transformations: a. b. c. d. e. Pyridine CI
-
Propose a plausible mechanism for each of the following reactions: a. b. stitl. [H,SO,] Conc. H2SO4
-
Assume that a CPA has just received a new audit client. The client will be the firms largest audit client, and the firm will have to hire one new staff member to staff the engagement. The fees will...
-
Pretty Pillows, Mfg., manufactures silk throw pillows. Last month the company produced 3,890 pillows. Using job order costing, determine the product unit cost for one pillow based on the following...
-
Approximate the following integrals by the midpoint rule, the trapezoidal rule, and Simpsons rule. Then, find the exact value by integration. Express your answers to five decimal places. LAVA xV4-x...
-
Create a House of Quality for a product familiar to you and complete the House of Quality form. Pick at least three different brands of the product to make the competitive comparison. List at least...
-
A recent poll asked 16- to 21-year-olds whether or not they are likely to serve in the U.S. military. The following table, cross-classified by gender and race, reports the percentage of those polled...
-
The purpose of a Property Management System is to: Select one: a. Manage the entire lodging property operation by keeping all of the information regarding all departments in one computer system. b....
-
Jimmy owns a garden in which he has planted N trees in a row. After a few years, the trees have grown up and now they have different heights. Jimmy pays much attention to the aesthetics of his...
-
Draw a plausible mechanism for each of the following reactions: (a) (b) [H,SO4] -H20 [H,SO4] -H20
-
Predict the product of each of the following reactions: (a) (b) [H2SO4] excess MeOH -? -H20 [H,SO4] -H20
-
A drug is assumed to be effective with an unknown probability p. To estimate p the drug is given to n patients. It is found to be effective for m patients. The method of maximum likelihood for...
-
Factor completely. x10 2x5 +1
-
Which of the three essential financial statements is most important for your business for the feasibility plan? Explain your answer.
-
Module 05 Content Background Having a comprehensive understanding of curriculum models and approaches to early childhood education can give you an appreciation of the many options available to...
-
- 7 ( 3 x - 2 ) 2 find the derivative
-
The Reciprocal Method Solve the Simultaneous Equations: S1=170,000+(0.2*S2) S2=68,000+(0.2*S1) S1=170,000+[0.2*(68,000+0.2*S1)]
-
Solve each system using the substitution method. If a system is inconsistent or has dependent equations, say so. -5x + 2y = -2 x + 6y = 26
-
DEPARTMENT DATA EMPLOYEE DATA EmployeeNumber FirstName Mary Rosalie Richard George Alan 3 4 5 7 8 9 855555ES 12 13 14 15 16 17 Create the database tables in SQL or ACCESS: 18 19 20 PROJECT DATA Ken...
-
Given the following sets of atoms, write bond-line formulas for all of the possible constitutionally isomeric compounds or ions that could be made from them. Show all unshared electron pairs and all...
-
(a) Consider a carbon atom in its ground state. Would such an atom offer a satisfactory model for the carbon of methane? If not, why not? (b) Consider a carbon atom in the excited state: Excited...
-
Open computer molecular models for dimethyl ether, dimethylacetylene, and cis-1,2- ichloro-1,2-difluoroethene from the 3D Molecular Models section of the book's website. By interpreting the computer...
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


