Draw a plausible mechanism for each of the following transformations: (a) (b) (c) (d) Meo, OMe [H,SO4]
Question:
Draw a plausible mechanism for each of the following transformations:
(a)
![Meo, OMe [H,SO4] excess MeOH -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1606/7/3/1/5905fc4c746e5bb91606731590218.jpg)
(b)
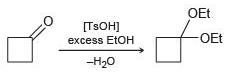
(c)
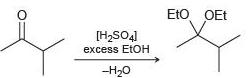
(d)
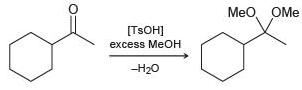
Transcribed Image Text:
Meo, OMe [H,SO4] excess MeOH -H20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
a b c d H H0 Me Me ...View the full answer

Answered By

Rohail Amjad
Experienced Finance Guru have a full grip on various sectors, i.e Media, Insurance, Automobile, Rice and other Financial Services.
Have also served in Business Development Department as a Data Anlayst
4.70+
32+ Reviews
83+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw a plausible mechanism for each of the following transformations: a. b. c. d. e. Pyridine CI
-
Propose a plausible mechanism for each of the following reactions: a. b. Br Br2 . [H,SO,]
-
Propose a plausible mechanism for each of the following transformations. a. b. c. d. e. f. 1) EtMgBr 2) - 1) NaH OEt 2) EtI
-
Listing 8.4 checks whether a solution is valid by checking whether every number is valid in the board. Rewrite the program by checking whether every row, every column, and every small box has the...
-
PRICE MART offered a whopping 10% discount on the average price of TV Plasma for fathers day. Customer response was so enthusiastic that unit sales rose by 15% over the previous month's level, a....
-
The following integrals cannot be evaluated in terms of elementary antiderivatives. Find an approximate value by Simpsons rule. Express your answers to five decimal places. S V + x dx; n = 4 3
-
In problem 16.6, what is the Cpk when: a. The process is centered on .75? Is the process capable? b. The process is centered on .74? Is the process capable? LO.1
-
A local TV station claims that 60% of people support Candidate A, 30% support Candidate B, and 10% support Candidate C. A survey of 500 registered voters is taken. The accompanying table indicates...
-
During 2021 Mary Beth, a cash basis taxpayer, received the following: 1. A gift from her brother $ 2,000.00 2. Dividends on her stock $500.00 3. A prize from a contest: a watch valued at $150.00 4....
-
Elsas financial year ends on 31 March. She depreciates her office equipment at 20% per annum on cost. Depreciation is calculated from the date of purchase. On 1 April the balances in Elsas books...
-
For most ketones, hydrate formation is unfavorable, because the equilibrium favors the ketone rather than the hydrate. However, the equilibrium for hydration of hexafluoroacetone favors formation of...
-
Draw a plausible mechanism for each of the following reactions: (a) (b) [H,SO4] -H20 [H,SO4] -H20
-
Discuss how world leaders use nationalism to pursue their goals both for personal and national gain. Discuss what purpose political leaders use nationalism.
-
1. (5 pts) Given y[n]= 2y[n-1] and y[0]=2, Write MATLAB code to calculate and plot y for 0
-
F ( t ) = t 4 + 1 8 t 2 + 8 1 2 , g ( t ) = ( t + 3 ) / 3 ; find ( f o g ) ( 9 )
-
How did they calculate allocated cost FLIGHT A FLIGHT 350 615 FLIGHT 3 1 Go GALS 20 G EXISTING SCHEME, DETERMINE THE OVE OR FLIGHTS A, B, AND C. 2 ED AT 7.00 PER K1.00 OF PILOT SALAF TOTAL NON-SALARY...
-
High Tech ManufacturingInc., incurred total indirect manufacturing labor costs of $540,000. The company is labor-intensive. Total labor hours during the period were 5,000. Using qualitativeanalysis,...
-
Start with AS/AD and IS/MP in full employment equilibrium. Assume the is a massive positive aggregate demand shock. How would this affect AS/AD and IS/MP and prices and output relative to the full...
-
If the rate of a plane in still air is x mph and the rate of a steady wind is 20 mph, what is the rate of the plane in each case? (a) The plane is flying into the wind (that is, into a headwind,...
-
Find the radius of convergence of? 1.2.3 1.3.5 (2n-1) r2n+1 -1
-
Draw a three-dimensional orbital representation for each of the following molecules, indicate whether each bond in it is a s or p bond, and provide the hybridization for each non-hydrogen atom. (a)...
-
Ozone (O3) is found in the upper atmosphere where it absorbs highly energetic ultraviolet (UV) radiation and thereby provides the surface of Earth with a protective screen. One possible resonance...
-
Write resonance structures for the azide ion, N3-. Explain how these resonance structures account for the fact that both bonds of the azide ion have the same length.
-
Show that the convexity for a zero coupon bond with m payments per year is (m) n(n + -)(1+ m m
-
Abdul Canarte , a Central Bank economist, noticed that the total group purchasing basket of goods (CPI) has gone from $149,740.00 to $344,460.00 in 8 years. With monthly compounding, what is the...
-
ABC Corporation expects sales next year to be $50,000,000. Inventory and accounts receivable (combined) will increase $8,000,000 to accommodate this sales level. The company has a profit margin of 6...

Study smarter with the SolutionInn App


