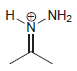
Draw all lone pairs on each of the nitrogen atoms in the compounds below. First, review in
Question:
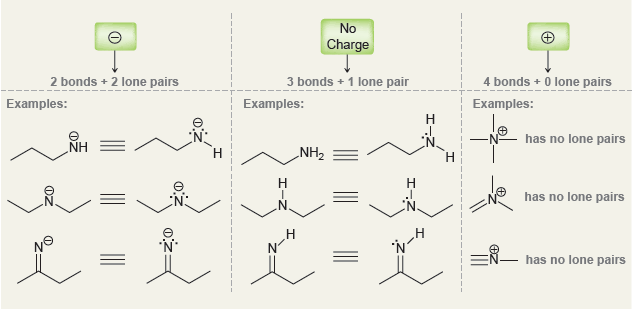
a.
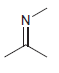
b.
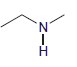
c.
d.
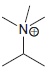
e.
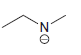
f.
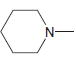
g.
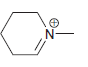
h.
Transcribed Image Text:
No Charge 3 bonds + 1 lone pair 2 bonds + 2 lone pairs 4 bonds + 0 lone pairs Examples: Examples: Examples: н has no lone pairs NH NH2 has no lone pairs .N' has no lone pairs z:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
a b c d N...View the full answer

Answered By

Muhammad Khurram
I have strong General Management skills to apply in your projects. Over last 3 years, I have acquired great knowledge of Accounting, Auditing, Microsoft Excel, Microsoft PowerPoint, Finance, Microsoft Project, Taxation, Strategic Management, Human Resource, Financial Planning, Business Planning, Microsoft Word, International Business, Entrepreneurship, General Management, Business Mathematics, Advertising, Marketing, Supply Chain, and E-commerce. I can guarantee professional services with accuracy.
4.80+
249+ Reviews
407+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw all lone pairs on each of the oxygen atoms in the compounds below. Before doing this, review in the following table, and then come back to these problems. Try to identify all lone pairs without...
-
The azide ion, N3-, is linear with two N-N bonds of equal length, 1.16 Ã. (a) Draw a Lewis structure for the azide ion. (b) With reference to Table 8.5, is the observed bond length consistent...
-
The following compound has three nitrogen atoms: Each of the nitrogen atoms exhibits a lone pair that can function as a base (to abstract a proton from an acid). Rank these three nitrogen atoms in...
-
If the owner of a company takes merchandise for personal use, what account is debited? a. Owners capital b. Owners withdrawals c. Purchases d. Cash
-
Per the Kiobel case, why might one disagree with the Court's Majority decision? Persuasively and rigorously explain.
-
Use a computer algebra system to approximate the iterated integral. 2 z+ 4 dz dr de Jo Jo Jo v
-
3. The proprietary fund statement of cash flows includes all of the following sections except: a Cash flows from operating activities b Cash flows from investing activities c Cash flows from capital...
-
Identifying suitable market segments and selecting targets are critical to the success of any marketing plan. As Jane Melodys assistant, youre responsible for market segmentation and targeting. Look...
-
Following are selected transactions for Vitalo Company Nov. 1 Accepted a $6,000, 180-day, 8 note dated November 1 from Kelly White in granting a tine extension on her past- due account receivable....
-
The Jolson Corporation produces 1,000 wood cabinets and 500 wood desks per year, the total cost being $30,000. If the firm produced 1,000 wood cabinets only, the cost would be $23,000. If the firm...
-
A carbene is a highly reactive intermediate in which a carbon atom bears a lone pair and no formal charge: How many hydrogen atoms are attached to the central carbon atom above?
-
Each of the following compounds contains both oxygen and nitrogen atoms. Identify all lone pairs in each of the following compounds: a. b. c. d. e. f. N. O=C=N
-
How can we determine the extent to which IS/IT services should be outsourced?
-
Provide a brief bio of the leader and a brief overview of the change or crisis they led the organization or movement through. Discuss their leadership style during this change/crisis using one of the...
-
Write a C++ function named Ifsr that accepts feedback path and initial states as unsigned integers and the number of random bits to be printed as arguments. The function will print the random bits by...
-
Newfoundland Hapset will be remitted to _____
-
The Z Energy Corp. has a new investment opportunity that generates cash flows of $6 million per year (in expectation) forever. The managers of Z Energy are not sure what the required rate of return...
-
Define an interface TwoStrings Oper declaring a function apply which takes two strings and returns a string. Then, define four classes implementing this interface, where the operation on strings...
-
Solve each system using the substitution method. If a system is inconsistent or has dependent equations, say so. 1 -X 1 4 5." 5x - y = 0 = 9
-
Find the APR in each of the following cases: NUMBER OF TIMES COMPOUNDED Semiannually Monthly Weekly Infinite EAR APR 10.4% 8.9 11.6 15.4
-
Propose structures for compounds E-H. Compound E has the molecular formula C5H8 and is optically active. On catalytic hydrogenation E yields F. Compound F has the molecular formula C5H10, is...
-
Consider the interconversion of cis-2-butene and trans-2-butene. (a) What is the value of Ho for the reaction cis-2-butene : trans-2-butene? (b) Assume Ho Go. What minimum value of DG would you...
-
(a) Partial dehydrohalogenation of either (1R, 2R)-1,2-dibromo-1,2-diphenylethane or (1S, 2S)-1,2-dibromo-1,2-diphenylethane enantiomers (or a race mate of the two) produces...
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


