For each of the following compounds, use the nitrogen rule to determine whether the molecular weight should
Question:
a.
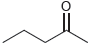
b.
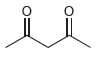
c.
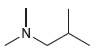
d.
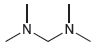
Transcribed Image Text:
O: O:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
a This compound does not have any nitrogen atoms Acco...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write the chemical formula for each of the following compounds, and indicate the oxidation state of nitrogen in each: (a) Sodium nitrite (b) Ammonia (c) Nitrous oxide (d) Sodium cyanide (e) Nitric...
-
For each of the following compounds, draw the important resonance forms. Indicate which structures are major and minor contributors or whether they have the same energy. (a) H2CNN (b) H2C=CH-NO2 (c)...
-
For each of the following compounds and ions, 1. Draw a Lewis structure. 2. Show the kinds of orbitals that overlap to form each bond. 3. Give approximate bond angles around each atom except...
-
Sedona Company set the following standard costs for one unit of its product for 2015. Direct material (20 Ibs. @ $2.50 per Ib.) . . . . . . . . . . . . . . . . . . . . . . . $ 50 Direct labor (10...
-
Implement the contains routine for MyLinkedList.
-
The measurement of the edge of a cube is found to be 15 inches, with a possible error of 0.03 inch. (a) Use differentials to approximate the possible propagated error in computing the volume of the...
-
Name two methods for identifying useful segments.
-
What will be the nominal rate of return on a preferred stock with a $100 par value, a stated dividend of 8 percent of par, and a current market price of? (a) $60, (b) $80, (c) $100, and (d) $140?
-
straight - line amortization method. What is the journal entry for the interest payment made on December 3 1 , 2 0 2 5 ? A . Cash 2 , 5 0 0 Interest Expense 2 , 5 0 0 B . Interest Expense Premium on...
-
The following selected transactions relate to liabilities of Interstate Farm Equipment Company for December 2024. Interstates fiscal year ends on December 31. Required: Prepare the appropriate...
-
Economists call the reduction in consumer confidence that resulted from the September 11 th attacks an _____ shock which leads to the ________. a. Aggregate demand, aggregate demand curve shifting...
-
For developed economies sustained increases in aggregate supply, absent increases in aggregate demand, will result in a. Growth for a while, but ultimately it will result in only inflation. b....
-
Identify the x- and y-intercepts of the line. Use the intercepts to write an equation of the line. y 1 X
-
The employee.class.php file contains an abstract, base class named Employee . One of the attributes of the class is an object of Person . This demonstrates one of the three relationship types among...
-
A direct shear test is performed on a saturated specimen of loose sand. A normal stress equal to 100 kPa is applied and a maximum shear stress of 75 kPa is measured in the shear test. Determine the...
-
How does budgeting help managers? Budgeting helps managers determine if their goals are ethical and achievable. Budgeting helps managers determine if their goals are reasonable and achievable....
-
If the 230-lb block is released from rest when the spring is unstretched, determine the velocity of the block after it has descended 5ft . The drum has a weight of 70lb and a radius of gyration of...
-
A B C D E F 1 Frequency : Monthly 2 Loan Amount: 150000.00 3 Interest Rate: 7.25% 4 Term(years): 30.00 5 No. of payments in a year: 12 time(s) 6 Periodic Rate: 0.60% =C3/C5 7 Total number of payment:...
-
Simplify each expression. Assume that all variables represent nonzero real numbers. 5 4 -2
-
An Atomic Energy Commission nuclear facility was established in Hanford, Washington, in 1943. Over the years, a significant amount of strontium 90 and cesium 137 leaked into the Columbia River. In a...
-
What aromatic products would you obtain from the KMnO4 oxidation of the followingsubstances? (a) O2N. (b) C(CH3)3 CH(CH3)2 "
-
Refer to Table 5.3 for quantitative idea of the stability of a benzyl radical. How much more stable (in kJ/mol) is the benzyl radical than a primary alkyl radical? How does a benzyl radical compare...
-
Styrene, the simplest alkenylbenzene, is prepared commercially for use in plastics manufacture by catalytic dehydrogenation of ethylbenzene. How might you prepare styrene from benzene using reactions...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


