For each pair of compounds below, determine whether they are identical compounds, constitutional isomers, stereoisomers, or different
Question:
(a)
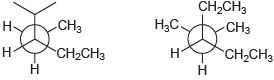
(b)
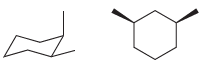
(c)
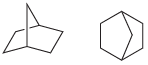
(d)
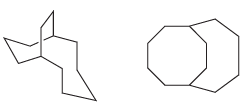
(e)
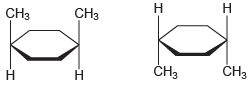
(f)
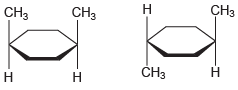
(g)
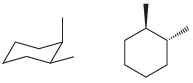
(h)
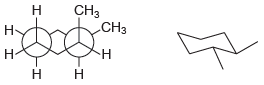
(i)
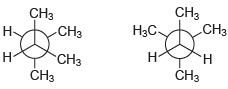
(j)
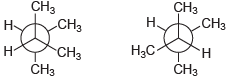
(k)

(l)
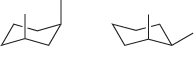
Transcribed Image Text:
CH,CH3 -CНз CHз H3C. Н- CH-CHз CH2CH3 Н Н Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
a Identical compounds b Constitutional isomers c Identi...View the full answer

Answered By

Poonam Chaudhary
I have 15 month+ Teaching Experience
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each pair of compounds below, identify the more acidic compound: (a) (b) (c) (d) (e) (f) (g) (h) SH
-
For each pair of compounds below, identify the more acidic compound: (a) (b) (c) (d) (e) (f) (b) C (d) H. -S -3- (f) X H' `H.
-
For each pair of compounds below, identify the stronger base: (a) (b) (c) (d) (e) (f) -
-
1) A survey of 200 public universities indicated that the 25th percentile of the yearly tuition cost of the universities was $4600 and the 75th percentile was $7100. The minimum value was $2000, the...
-
Explain the uses for each of the three classifications of ratios: liquidity, solvency, and profitability. Calculate the current ratio, profit margin, and after tax ROE, of a real company whose...
-
If the 75-kg crate starts from rest at A, and its speed is 6m/s when it passes point B, determine the constant force F exerted on the cable. Neglect friction and the size of the pulley. A -6 m- B -2...
-
Salary for clerical staff is a (a) Indirect expense (b) Indirect labour (c) Indirect material (d) Direct expense
-
City Place Movie Theaters has four employees and pays them on an hourly basis. During the week beginning June 24 and ending June 30, 2019, these employees worked the hours shown below. Information...
-
Please submit the solutions to the following tasks in the same sequence as they appear on the test through the Assignment Link. For this part of the test you will need the AP_Trans (Accounts_Payable...
-
On a late November morning in 2012, a boutique investment bank in Japan approached Mr. Takuya Saito, founder and CEO of Saito Solar, about his interest in selling the rm. Even though selling the rm...
-
Do you expect cyclohexene to adopt a chair conformation? Why or why not? Explain. Cyclohexene
-
Predict the major product(s) for each of the following reactions: 1) Hg(OAC)2, -0 2) NABH, ? - O, NaOH, cold Br2 H20 Pt
-
A stock price is governed by geometric Brownian motion with \(\mu=.20\) and \(\sigma=.40\). The initial price is \(S(0)=1\). Evaluate the four quantities E[In S(1)], E[S(1)], stdev[In S(1)]...
-
Service provides commercial and industrial appraisals and feasibility studies. On January 1 , the assets and liabilities of the business were the following: Cash, \(\$ 8,700\); Accounts Receivable,...
-
Sketch the mapping of the value chain for: a A consulting firm b An airline c A trading firm d A corporate and investment bank e An internet-based platform (e.g. Airbnb, Netflix)?
-
Red River Banking Company has ten automatic i) AND teller machines (ATMs) spread throughout the city maintained by the ATM Department. You have been assigned the task of determining the cost of...
-
Super Day Spa provided \(\$ 120,000\) of services during 2012. All customers paid for the services with credit cards. Super submitted the credit card receipts to the credit card company immediately....
-
The following data represent the height of 26 statistics students as measured in inches: a. Create a frequency table for these data. b. Create a histogram for these data with an interval width of 1...
-
In Exercises 5358, begin by graphing f(x) = log 2 x. Then use transformations of this graph to graph the given function. What is the vertical asymptote? Use the graphs to determine each functions...
-
The Adjusted Trial Balance columns of a 10-column work sheet for Webber Co. follow. Complete the work sheet by extending the account balances into the appropriate financial statement columns and by...
-
Explain which of the two methyl esters, product 1 or product 2, is formed when the alkyl chloride is heated in methanol. Problems using online three-dimensional molecular models
-
Explain which of these two alkyl chlorides reacts faster with sodium acetate in DMSO. Problems using online three-dimensional molecular models
-
Arrange these nucleophiles in order of decreasing rate of reaction with iodomethane and explain your answer. Problems using online three-dimensional molecular models
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer

Study smarter with the SolutionInn App


