Guanidine is a neutral compound but is an extremely powerful base. In fact, it is almost as
Question:
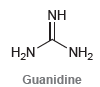
Transcribed Image Text:
NH H,N NH2 Guanidine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
Protonation of the nitrogen highlighted below ...View the full answer

Answered By

Anoop V
I have five years of experience in teaching and I have National Eligibility in teaching (UGC-NET) .
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Histidine possesses a basic side chain which is protonated at physiological pH. Identify which nitrogen atom in the side chain is protonated.
-
Clomipramine is marketed under the trade name Anafranil and is used in the treatment of obsessive compulsive disorder. (a) Identify which nitrogen atom in clomipramine is more basic, and justify your...
-
Arginine is the most basic of the 20 naturally occurring amino acids. At physiological pH, the side chain of arginine is protonated. Identify which nitrogen atom in the side chain is protonated.
-
A bartender employed in a licensed establishment over-serves a patron. As a result of the over-service, the patron physically assaults another patron by striking him with a beer bottle. Identify and...
-
In 2015, there were approximately 8.3 million unemployed workers in the United States. The circle graph shows the age profile of these unemployed workers. (a) Estimate the number of unemployed...
-
You are a junior investment associate at Number One National Bank, a top-tier Canadian bank. It is your first day on the job and your manager asks you the following question regarding investments:...
-
How does treasury stock affect the authorized, issued, and outstanding shares? AppendixLO1
-
Various types of accounting changes can affect the auditor's report. a. Briefly describe the rationale for having accounting changes affect the auditor's report and the auditor's responsibility in...
-
Garden Depot is a retailer that is preparing its budget for the upcoming fiscal year Management has prepared the following summary of its budgeted cash flows Total cash receipts Total cash...
-
1. How comparable are the two different methods? In what ways are they similar? In what ways are they different? 2. What are the positive and negative aspects of each approach that Shocker should...
-
Propose an efficient synthesis for each of the following compounds using the acetoacetic ester synthesis. (a) (b) (c) (d)
-
Complete a situation analysis for Fork and Dagger, highlighting possible order qualifiers and order winners for the food and hospitality industry in Struan.
-
Classify the traditional sources of risk as to whether they are general sources of risk or specific sources of risk.
-
Steve Reese is a well-known interior designer in Fort Worth, Texas. He wants to start his own business and convinces Rob ODonnell, a local merchant, to contribute the capital to form a partnership....
-
One of the main purposes of evaluation research is: a. reexamining previously collected data. b. monitoring and improving programs. c. generating rich descriptions of individual perspectives. d....
-
10.13 Sweetlip Ltd and Warehou Ltd are two family-owned flax-producing companies in New Zealand. Sweetlip Ltd is owned by the Wood family and the Bradbury family owns Warehou Ltd. The Wood family has...
-
distribution that is skewed to the right instead of being normally distributed. Assume that we collect a random sample of annual incomes of 50 statistics students. Can the distribution of incomes in...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
Solve for y: Ax + By = Cy + D.
-
The column shown in the figure is fixed at the base and free at the upper end. A compressive load P acts at the top of the column with an eccentricity e from the axis of the column. Beginning with...
-
There are eight different five-carbon alkyl groups. (a) Draw them. (b) Give them systematic names. (c) In each case, label the degree of substitution (primary, secondary, or tertiary) of the head...
-
Use a Newman projection, about the indicated bond, to draw the most stable conformer for each compound. (a) 3-methylpentane about the C2-C3 bond (b) 3, 3-dimethylhexane about the C3-C4 bond
-
(a) Draw the two chair conformations of cis-1, 3-dimethylcyclohexane and label all the positions as axial or equatorial. (b) Label the higher-energy conformation and the lower-energy conformation....
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


