How would you distinguish between each pair of compounds using high-resolution mass spectrometry? a. b. .
Question:
a.
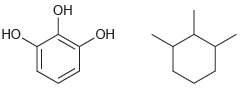
b.
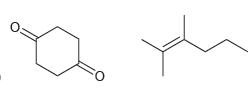
Transcribed Image Text:
Он Но. но
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
a b OH ...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How would you distinguish between each pair of compounds in Problem 15.29 using IR spectroscopy? Problem 15.29 a. b. OH HO. HO m/z = 126.0315 m/z = 126.1404
-
How would you distinguish between each pair of compounds using mass spectrometry? a. b. N.
-
How would you distinguish between the following compounds using 13 C NMR spectroscopy?
-
One end of a light, elastic string, of natural length 1.2m and modulus of elasticity 32N, is attached to a fixed point, B. A particle, P, of mass 1.5 kg, is then attached to the other end of the...
-
On July 1,2015, Walter Allen Inc. purchased 6,000 shares of the outstanding common stock of Piaffe Corporation at a cost of $140,000. Piafee had 30,000 shares of outstanding common stock. Assume the...
-
Refer to the Sportade example in Appendix 3A and the data and your answer to S3-16. Data in S3-16. Consider the Sportade example in Appendix 3A . Suppose that during April, the company produces...
-
3. What fund financial statements are needed for an enterprise fund to meet the requirements for fair presentation in accordance with GAAP? Which government-wide statements include enterprise fund...
-
The M-form structure enables firms to pursue complex corporate diversification strategies by delegating different management responsibilities to different individuals and groups within a firm. Based...
-
The Grape Vine Market is a nearly 1,700-square-metre store offering highly collectible food and wine-along with some other culinary experiences, including a cooking school-all in a setting crafted to...
-
The dam for a lake is designed to withstand the additional force caused by silt that has settled on the lake bottom. Assuming that silt is equivalent to a liquid of density ps = 1.76 Ã 103...
-
The following are mass spectra for the constitutional isomers ethylcyclohexane and 1, 1-dimethylcyclohexane. Based on likely fragmentation patterns, match the compound with its spectrum. 100 100 80 -...
-
An aldehyde with molecular formula C 4 H 6 O exhibits an IR signal at 1715 cm 1 . (a) Propose two possible structures that are consistent with this information. (b) Describe how you could use 13 C...
-
Define OLAP, and give some examples.
-
4. Thinking Ahead (2 points): Project 1 involves the analysis of a bicycle pedal. Consider the bicycle shown below. If a rider places their full weight on the pedal when it is in the horizontal...
-
On January 1, 2023, Martineau Corp. issued a 5-year, 5% installment note payable for $118,000 to finance upgrading its current equipment. The company's year end is December 31. The repayment of...
-
Multiply. 2 x-x-2 3x-3 2 x+2x-3 x+1 Simplify your answer as much as possible.
-
Explain the processes of querying a relational database and define Big Data and explain its basic characteristics. Compare and contrast the major types of networks. - Identify the fundamentals of...
-
42. Explain why the inequality x - x + 1 < 0 has the empty set as the solution set.
-
Point A has coordinates (-2, 6) and point B has coordinates (4, -2). What is the equation of the vertical line through B?
-
Use this circle graph to answer following Exercises. 1. What fraction of areas maintained by the National Park Service are designated as National Recreation Areas? 2. What fraction of areas...
-
Open the computer molecular model titled "1-Bromo [2.2.1] bicycloheptane LUMO" at the book's website for the lowest unoccupied molecular orbital (LUMO) of this compound. Where is the lobe of the LUMO...
-
In the previous problem and the associated molecular model at the book's website, you considered the role of HOMOs and LUMOs in an SN2 reaction. (a) What is the LUMO in an SN1 reaction and in what...
-
SN2 reactions that involve breaking a bond to a chirality center can be used to relate configurations of molecules because the stereochemistry of the reaction is known. (a) Illustrate how this is...
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


