Identify each of the following compounds as aromatic, nonaromatic, or antiaromatic. Explain your choice in each case.
Question:
a.
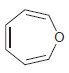
b.
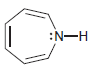
c.
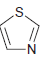
d.
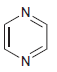
e.
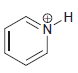
f.
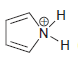
g.
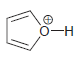
h.
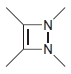
Transcribed Image Text:
:N-H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
a Nonaromatic The lone pairs on the oxygen atom will remain in sp 3 hybridized orbitals in order to ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify each of the following compounds as a fat or an oil. Explain your answers. (a) A triglyceride containing one palmitic acid residue and two stearic acid residues (b) A triglyceride containing...
-
Identify each of the following compounds from their spectra. (a) Compound A: molecular mass 113; gives a positive hydroxamate test; IR 2237, 1733, 1200 cm-1; proton NMR: 1.33 (3H, t, J = 7 Hz), ...
-
Identify each of the following compounds from the NMR data and molecular formula. The number of hydrogens responsible for each signal is shown in parentheses. a. C4H8Br2 1.97 ppm (6) singlet 3.89 ppm...
-
Tabulate the function f(x) = sin x for x = 0.0(0.2)1.6. From this table estimate, by linear interpolation, the value of sin 1.23. Construct a table equivalent to Figure 2.102, and so estimate the...
-
What is a domestic joint venture? A foreign joint venture? What advantages does taking on an international partner through a joint venture offer? What are the disadvantages? (LO 3: Explain how to...
-
Determine the center and radius of each circle. Sketch each circle. x 2 + y 2 + 4.20x 2.60y = 3.51
-
19. What is included on a budgetary comparison schedule? Is such a schedule required to be included in a CAFR?
-
Explain why functional currency should be re-measured, rather than translated, when a foreign entitys functional currency is highly inflationary.
-
2.5points eBook Print References Check my workCheck My Work button is now enabled Item 4 Item 4 2.5 points Bonnie Denim Company sells blue jeans. Last year, skinny jeans were fashionable; this year,...
-
A large tank is filled to capacity with 500 gallons of pure water. Brine containing 2 pounds of salt per gallon is pumped into the tank at a rate of 5 gal/min. The well-mixed solution is pumped out...
-
Firefly luciferin is the compound that enables fireflies to glow. a. The structure exhibits three rings. Identify which of the rings are aromatic. b. Identify which lone pairs are participating in...
-
Describe several cases when a periodic review model would be preferred to a ROP model.
-
Refer to the information provided in problem 8.23. Required (a) Prepare a process cost report using the FIFO method. (b) Explain how a standard cost report would differ from the FIFO report you just...
-
Absorption linewidth for an absorbing atomic transition. Consider the curves of power transmission T(w) = exp[-2am(w)L] through an atomic medium with a lorentzian resonant transition, plotted versus...
-
EXAMPLE 05.04 Z Write the force and the couple in the vector form (with rectangular/Cartesian components). Use C = 180 N-m and P = 500 N O INDIVIDUAL Submission (IS12) D x 400 mm B C 300 mm A 400 mm...
-
1.XYZ Corporation budgets factory overhead cost of P500,000 for the coming year. Compute for the overhead cost applied to the job. The following data are available: Budgeted annual overhead for...
-
OP Technologies Manufacturing manufactures small parts and uses an activity-based costing system. Activity Materials Assembling Packaging Est. Indirect Activity Costs $65,000 $242,000 $90,000...
-
3. Solve Example 3.7 (Bergman, Lavine, Incropera, and DeWitt, 6th Ed., pp. 129-132, or 7th Ed., pp. 145-149, or 8th Ed., pp. 134-138), but use the finite difference method. T T = 30C Insulation-...
-
Hearty Soup Co. uses a process cost system to record the costs of processing soup, which requires the cooking and filling processes. Materials are entered from the cooking process at the beginning of...
-
DC has unused FTC carryover from 2017 in the separate category for GC income as the result of income generated by a foreign branch. The income was foreign source general category income. In 2018 the...
-
The chlorohydrins trans - 2 chlorocyclohexanol reacts rapidly in base to form an epoxide. The cis steroisomer, however, is relatively unreactive and does not give an epoxide. Explain why the two...
-
Explain the following facts with a mechanistic argument. (a) When the reaction mixture in part (a) is heated for times, l-iodobutane is also formed.
-
What products are formed when gach of the following ethers reacts with concenffated aqueous HI? 2-ethoxy-2,3-dimethylbutane
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


