Identify reagents that can be used to achieve each of the following transformations: a. b. c. Br
Question:
a.
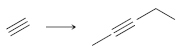
b.
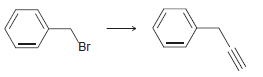
c.

Transcribed Image Text:
Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
a b c ...View the full answer

Answered By

Mahima Bawa
I have more than 2 Years of experience as a subject matter expert .I have attained quality knowledge in different realms of Statistics and Actuarial Science reflective in my grades and have a bit of practical experience through distinctive professional and volunteering engagements.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the reagents necessary to achieve each of the following transformations: Br Br - " Br Br "Br
-
Identify the reagents necessary to achieve each of the following transformations: Br Br Br Br Br
-
Identify the reagents necessary to achieve each of the following transformations: (a) (b) (c) (d) (e) (f) HO H.
-
A money market portfolio has a market value of $20,000,000 and its value will change by $500 for a change in short-term yields of one basis point. The eurodollar futures contract has a tick size of...
-
Write a Case Notes for "OFold case"
-
The force exerted by the motor M on the cabilses hown in the graph. Determine the velocity of the 200-lb crate A when t = 2.5 s. M 250 lb FO A F (lb) 2.5 t (s)
-
Define opportunity cost.
-
State the effect (cash receipt or payment and amount) of each of the following transactions, considered individually, on cash flows: a. Retired $500,000 of bonds, on which there was $5,000 of...
-
A line passes through the point (3,3) and has a slope of -4 . Write an equation in point-slope form for this line
-
Slater & Gordon (S&G) became the worlds first listed law firm in 2007. The company is headquartered in Melbourne, where it was founded in 1935, by William Slater and Hugh Gordon....
-
Draw a Lewis structure for each of the following compounds: (a) C 2 H 6 (b) C 2 H 4 (c) C 2 H 2 (d) C 3 H 8 (e) C 3 H 6 (f) CH 3 OH
-
Borane (BH 3 ) is very unstable and quite reactive. Draw a Lewis structure of borane and explain the source of the instability.
-
Setting the decision rule for one-way, repeated-measures ANOVA. (Assume = .05.) Given df Treatment = 3 and df Residual = 36, (a) What is F cv ? (b) Draw the sampling distribution of F, being sure to...
-
The Buckle, Inc., operates 387 stores in 39 states, selling brand name apparel like Lucky jeans and Fossil belts and watches. Some of the items included in its 2008 statement of cash flows presented...
-
Assume that on July 1, 2011, Big Corp. loaned Little Corp. \(\$ 12,000\) for a period of one year at 6 percent interest. What amount of interest revenue will Big report for 2011? What amount of cash...
-
A vacuum column with 25 real stages is operating with a pressure drop of \(0.3 \mathrm{in}\). of water per stage. Assume pressure drop in the condenser and the reboiler is 0.6 in. of water each. The...
-
You want to determine the viscosity of an oil which has an SG of 0.9. To do this, you drop a spherical glass bead $(\mathrm{SG}=2.7)$ with a diameter of $0.5 \mathrm{~mm}$ into a large vertical...
-
Show that 673 - 356 can be computed by adding 673 to the 10's complement of 356 and discarding the end carry. Draw the block diagram of a three-stage decimal arithmetic unit and show how this...
-
In Exercises 4170, use properties of logarithms to condense each logarithmic expression. Write the expression as a single logarithm whose coefficient is 1. Where possible, evaluate logarithmic...
-
Define a traverse in Surveying?
-
Show the products of these elimination reactions and indicate which ismajor: OTs . b) CH,OH + CH,0 + OH ELOH CI E:OH + CH,CH,O c)
-
The reaction of 2-bromobutane with ethoxide ion in ethanol gives 81% of a mixture of (Z)- and (E)-2-butene. Explain which stereo isomer you expect to predominate in this mixture.
-
Show the products of these reaction and indicate which ismajor: N(CH3)3 N(CH3)3 a) b) d) c) CH3 .
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


