Identify the hybridization state and geometry of each carbon atom in the following compounds: a. b. c.
Question:
a.
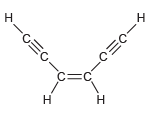
b.
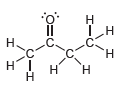
c.
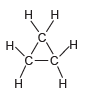
Transcribed Image Text:
Н н -OEJ- -CEC-C Н. .C. H. н1 H' н Н т.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
a The highlighted carbon atoms are sp 2 hybridized and trigonal pla...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the hybridization state and geometry of each carbon atom in benzene. Use that information to determine the geometry of the entire molecule: .C: H. .C. Benzene
-
Nicotine is an addictive substance found in tobacco. Identify the hybridization state and geometry of each of the nitrogen atoms in nicotine: C-H N. C- H. Nicotine z: I I-O
-
Melatonin is an animal hormone believed to have a role in regulating the sleep cycle: The structure of melatonin incorporates two nitrogen atoms. What are the hybridization state and geometry of each...
-
There is a mathematical puzzle called "Four Fours." In it, you use four 4s to make other whole numbers. Examples: You can make 61 (4+4)+4+4-6 Four Fourths You can also make 9! You Try! What other...
-
Would you advocate background music at the workstation? What results would you anticipate?
-
Kim says she thinks President Donald Trump is doing a good job. Coletta starts talking politics and how much she hates President Trump. Identify the political behavior in each situation as A....
-
Game performance of water polo players. Refer to the Biology of Sport (Vol. 31, 2014) study of the physiological LO9 performance of top-level water polo players, Exercise 11.24 (p. 626). Recall that...
-
(a) Prepare any necessary transaction entries for 2019 and adjusting entries at December 31, 2019, using the financial statement effects template.(b) Prepare any necessary transaction entries for...
-
An unfavorable flexible-budget variance for variable costs may be the result
-
Snack That Sale and Leaseback Snack That Inc (Snack That) is a snack food and bakery product company with a large manufacturing and distribution facility in Evansville, Indiana Snack That frequently...
-
An attorney defending a whale meat supplier accused of improperly labeling meat would most likely claim that the inferences drawn from the prosecutions evidence were questionable. Provide a potential...
-
We will learn all of the following reactions in upcoming chapters. For each of these reactions, notice that the product is an anion (ignore the positively charged ion in each case). In order to...
-
A rolled Cu-30 wt% Zn plate 0.500-in.- thick has 2% elongation as-received by the supplier. The desired specifications for the final sheet are a thickness of 0.125 in., minimum tensile strength of...
-
Bybee Printing makes custom posters and is currently considering making large-scale outdoor banners as well. Which one of the following is the best example of an incremental operating cash flow...
-
https://filmsfortheearth.org/en/film/humans-destroyers-of-earth/ This documentary will give you more insight into the speed at which globalization has taken place during the dawn of...
-
1. Write and explain (through comment or description after the program) a complete C program to perform the following activities: a. Take Student ID as user input from keyboard (2) b. Determine the...
-
Assume that I have a Company uses a job costing system with machine hours as the allocation base for overhead. The company uses normal costing to develop the overhead allocation rate. The following...
-
(CASE STUDY )Company Information: ABC Company is a large automotive dealer company operating in the field of automobile retailing that is owned by a big Holding Group Company XYZ. ABC Company buys...
-
The following exercises are of mixed variety. Factor each polynomial. k 2 - 6k - 16
-
Briefly discuss the implications of the financial statement presentation project for the reporting of stockholders equity.
-
Ammonium cyanate is composed of an ammonium caution (NH 4 + ) and a cyanate anion (OCN ). Show a Lewis structure for the cyanate anion. (Both O and N are bonded to C.) Which atom has the negative...
-
(a) Show a Lewis structure for urea. CH 4 N 2 O. Both Ns and the O are bonded to the C. The Hs are bonded to the Ns. None of the atoms has a formal charge. (b) Show a Lewis structure of an isomer of...
-
Phosphorus forms two compounds with chlorine, PCl 3 , and PCl 5 . The former follows the octet rule, but the latter does not. Show Lewis structures for each of these compounds. For the corresponding...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...
-
Which of the following concerning short-term financing methods is NOT CORRECT? Short-term bank loans typically do not require assets as collateral. Firms generally have little control over the level...
-
Kingbird Corporation is preparing its December 31, 2017, balance sheet. The following items may be reported as either a current or long-term liability. 1. On December 15, 2017, Kingbird declared a...

Study smarter with the SolutionInn App


