Identify the reagents necessary to accomplish each of the following transformations: SO3H Br ZON i NH2
Question:
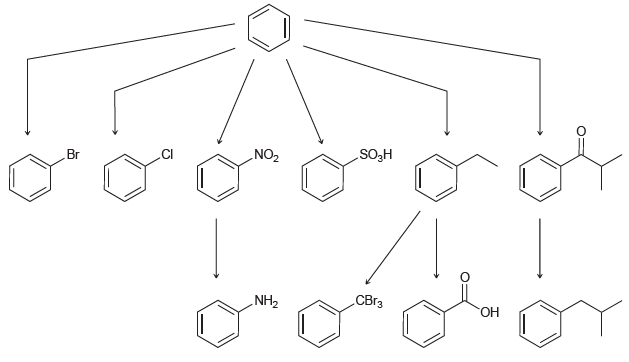
Transcribed Image Text:
SO3H Br ZON СВiз „NH2 ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (11 reviews)
CI AICI3 Fuming HSO4 EtCl A...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the reagents necessary to accomplish each of the transformations below: (a) (b) OH HO NH2
-
Identify the reagents necessary to accomplish each of the transformations below: (a) (b)
-
Identify the reagents necessary to accomplish each of the transformations below: (a) (b) Me
-
If = f() but the iteration x n+1 = f (x n ) fails to converge to the root a, under what condition on f(x) will the iteration x n+1 = f 1 (x n ) converge?
-
How does personal conflict affect partnerships? What steps might partners take to minimize personal conflict?
-
Determine the following inverse z-transforms using the partial fraction expansion method. a) X(z) = b) X(z) = 1-z-1 11 - 4z-2 +4z-3 13 1 - Z-1 + -2 z- - 8 -12-3 z33z24z+1 z34z2 +z -0.16
-
Test the hypothesis that the mean temperature of women is 98.6F. What can you conclude at a level of significance of a = 0.01?
-
With a smoothing constant of = 0.2, equation (6.2) shows that the forecast for the 13th week of the gasoline sales data from Table is given by F13 = 0.2Y12 + 0.8F12. However, the forecast for week...
-
I need helo regarding this task Question 10 Not yet answered A, B and C formed a partnership and agreed the following profit and loss agreement A will receive salaries of 100,000 and 5% bonus on...
-
Consider the following data on full-service restaurant sales. Calculate both the three-month and five-month moving averages for these data, and compare the forecasts by calculating the mean absolute...
-
Predict the change in the partial pressure of CO2 as a platinum catalyst is introduced into the reaction vessel at constant volume and temperature. CO(g) + 1/2O 2 (g) CO 2 (g) at equilibrium for...
-
Predict the change in the partial pressure of CO2 as O2 is removed from the reaction vessel at constant pressure and temperature. CO(g) + 1/2O 2 (g) CO 2 (g) at equilibrium for which H o R = 283.0...
-
What factors (limitations) enter into our evaluation of return on net operating assets?
-
The copper coil placed inside a stove with the purpose of heating water that flows through the coil. The coil is made from copper tube with an OD of 1 2 . 7 0 mm and ID of 1 1 . 0 8 mm . Water enters...
-
Confidence Levels Given specific sample data, such as the data given in Exercise 1, which confidence interval is wider: the 95% confidence interval or the 80% confidence interval? Why is it wider?
-
Yellow M&Ms Express the confidence interval (0.0847, 0.153) in the form of P - E < p < p + E. 12. Blue M&Ms Express the confidence interval 0.255 (+-) 0.046 in the form of P - E < p < p + E.
-
An ideal, noble gas with a mass of 97.2 g at 25 C and a pressure of 608 torr has a volume of 22.7 L. 1. What is the pressure (in atm)? SHOW ALL WORK. 2. What is R (number and units)? 3. What is the...
-
A drug is used to help prevent blood clots in certain patients. In clinical trials, among 4705 patients treated with the drug, 170 developed the adverse reaction of nausea. Construct a 95% confidence...
-
Suppose that a girl is 4 feet 6 inches at age 10. Explain how to use the function in Exercises 113114 to determine how tall she can expect to be as an adult. Data from exercise 113-114 The percentage...
-
After looking at the resources, explain what a spirit image is. Why might looking at a god and/or a human in terms of their spirit be helpful if you want to eliminate some of the divisions between...
-
In the following set, the NMR spectra of the compounds shown consist of a single resonance. Arrange the compounds in order of increasing chemical shift, smallest first. CH,CI, CH212 CH31
-
In the following set, the NMR spectra of the compounds shown consist of a single resonance. Arrange the compounds in order of increasing chemical shift, smallest first. CH,CI, CH212 CH31
-
Specify whether the labeled protons in each of the following structures would be expected to have the same or different chemical shifts. (a) (b) CH3 H,C CH3 HC C-CH CI Hb
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


