In Chapter 23, we will learn that treatment of ammonia with excess methyl iodide produces a quaternary
Question:
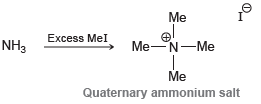
Transcribed Image Text:
Me Excess Mel NH3 MeN-Me Me Quaternary ammonium salt
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 37% (8 reviews)
H H H Me Me I MeNMe ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the products that are expected when -dgalactopyranose is treated with excess methyl iodide in the presence of silver oxide, followed by aqueous acid.
-
Physostigmine, an alkaloid obtained from a West African plant, is used in the treatment of glaucoma. Treatment of Physostigmine with methyl iodide gives a quaternary ammonium salt. What is the...
-
Physostigmine, an alkaloid obtained from a West African plant, is used in the treatment of glaucoma. Treatment of physostigmine with methyl iodide gives a quaternary ammonium salt. What is the...
-
Use the data in the table to complete the following. (a) Make a scatterplot of the data. Estimate a value for b so that f(x) = 0.0002x b models the data. (b) Check the accuracy of f(x). (c) The moon...
-
Identify how your literature review fits within a conceptual framework(s) within your specialization and describe the conceptual and/or theoretical frameworks used by the authors. Compare and...
-
Simplify the given expressions. cos 4 u sin 4 u
-
9. Given an 8-quarter oil swap price of $20.43, construct the implicit loan balance for each quarter over the life of the swap.
-
On January 1, 20--, a depreciable asset was acquired for $5,760. The asset has an estimated useful life of four years (48 months) and no salvage value . Use the straight-line depreciation method to...
-
with solution please Tatu Corp. makes all sales on credit. During May 2016, total credit sales were $2,650,000, collections were $2,400,000 and accounts written off as uncollectible were $25,000. The...
-
Jimmy owns a garden in which he has planted N trees in a row. After a few years, the trees have grown up and now they have different heights. Jimmy pays much attention to the aesthetics of his...
-
Draw the mechanism for each of the following solvolysis reactions: (a) (b) (c) (d) OMe (solvolysis) 'CI ETOH (solvolysis) Br
-
Identify whether each of the following substrates favors SN2, SN1, both, or neither: (a) (b) (c) (d) (e) (f) (g) -Br CI
-
a. Calculate the average daily change in the Index, shown to 6 decimal places. b. State the average daily change in the S&P 500 Index for the month of November 2012 as a percentage. c. State the...
-
Case Study : While it might be easy to see the negative effects on the environment from car emissions or the waste we produce, fewer people think about the effects of discarded clothes on the...
-
CompanyWeek 8 Assignment - Financial Statement Analysis Overview In this assignment, you will take your work with financial statements to the next level. You will analyze financial statements similar...
-
In Exercises 9-12, assume that 100 births are randomly selected. Use subjective judgment to describe the given number of girls as (a) significantly low, (b) significantly high, or (c) neither...
-
Which of the following is not included in the cash flow statement? a. Cash from short-term investments b. Cash from operations c. Cash from the balance sheet d. Cash from capital financing Which of...
-
Case Study Chapter 13B Pharm - Saved Case Study Chapter 13 Central Nervous System Stimulants and Related Drugs Nancy has been unsuccessful in preventing migraine headaches and has been prescribed a...
-
Solve the equation. Check your answers. 2x - 4 + 2 = 3x + 4
-
The cash records of Holly Company show the following four situations. 1. The June 30 bank reconciliation indicated that deposits in transit total $720. During July, the general ledger account Cash...
-
Give the structure of: 2E,7Z)-5-[(4- 1-propenyl]-2,7 -non adiene Be sure to read Study Guide Link 4.2 if you have difficulty with this problem.
-
Starting with the same two alkenes, would the products be different if DBr were used? Explain.
-
An alkene X with molecular formula C7H12 adds HBr to give a single alkyl halide I with molecular formula C7H13Br and undergoes catalytic hydrogenation to give 1,1-dimethylcyclopentane. Draw the...
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


